Ring-opening polymerization
A polymerization in which a cyclic monomer yields a monomeric unit which is acyclic or contains fewer cycles than the monomer. Note: If monomer is polycyclic, the opening of a single ring is sufficient to classify the reaction as ring-opening polymerization.
Penczek S.; Moad, G. Pure Appl. Chem., 2008, 80(10), 2163-2193

In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic.
Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative.[3] Many rings undergo ROP.[4]
Monomers
[edit]Many cyclic monomers are amenable to ROP.[5] These include epoxides,[6][7] cyclic trisiloxanes,[citation needed] some lactones[6][8] and lactides,[8] cyclic anhydrides,[7] cyclic carbonates,[9] and amino acid N-carboxyanhydrides.[10][11] Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization. Even highly strained cycloalkane rings, such as cyclopropane[12] and cyclobutane[13] derivatives, can undergo ROP.
History
[edit]Ring-opening polymerization has been used since the beginning of the 1900s to produce polymers. Synthesis of polypeptides which has the oldest history of ROP, dates back to the work in 1906 by Leuchs.[14] Subsequently, the ROP of anhydro sugars provided polysaccharides, including synthetic dextran, xanthan gum, welan gum, gellan gum, diutan gum, and pullulan. Mechanisms and thermodynamics of ring-opening polymerization were established in the 1950s.[15][16] The first high-molecular weight polymers (Mn up to 105) with a repeating unit were prepared by ROP as early as in 1976.[17][18]
An industrial application is the production of nylon-6 from caprolactam.
Mechanisms
[edit]Ring-opening polymerization can proceed via radical, anionic, or cationic polymerization as described below.[19] Additionally, radical ROP is useful in producing polymers with functional groups incorporated in the backbone chain that cannot otherwise be synthesized via conventional chain-growth polymerization of vinyl monomers. For instance, radical ROP can produce polymers with ethers, esters, amides, and carbonates as functional groups along the main chain.[19][20]
Anionic ring-opening polymerization (AROP)
[edit]
Anionic ring-opening polymerizations (AROP) involve nucleophilic reagents as initiators. Monomers with a three-member ring structure - such as epoxides, aziridines, and episulfides - undergo anionic ROP.[20]
A typical example of anionic ROP is that of ε-caprolactone, initiated by an alkoxide.[20]
Cationic ring-opening polymerization
[edit]Cationic initiators and intermediates characterize cationic ring-opening polymerization (CROP). Examples of cyclic monomers that polymerize through this mechanism include lactones, lactams, amines, and ethers.[21] CROP proceeds through an SN1 or SN2 propagation, chain-growth process.[19] The mechanism is affected by the stability of the resulting cationic species. For example, if the atom bearing the positive charge is stabilized by electron-donating groups, polymerization will proceed by the SN1 mechanism.[20] The cationic species is a heteroatom and the chain grows by the addition of cyclic monomers thereby opening the ring system.

The monomers can be activated by Bronsted acids, carbenium ions, onium ions, and metal cations.[19]
CROP can be a living polymerization and can be terminated by nucleophilic reagents such as phenoxy anions, phosphines, or polyanions.[19] When the amount of monomers becomes depleted, termination can occur intra or intermolecularly. The active end can "backbite" the chain, forming a macrocycle. Alkyl chain transfer is also possible, where the active end is quenched by transferring an alkyl chain to another polymer.
Ring-opening metathesis polymerization
[edit]Ring-opening metathesis polymerisation (ROMP) produces unsaturated polymers from cycloalkenes or bicycloalkenes. It requires organometallic catalysts.[19]
The mechanism for ROMP follows similar pathways as olefin metathesis. The initiation process involves the coordination of the cycloalkene monomer to the metal alkylidene complex, followed by a [2+2] type cycloaddition to form the metallacyclobutane intermediate that cycloreverts to form a new alkylidene species.[23][24]
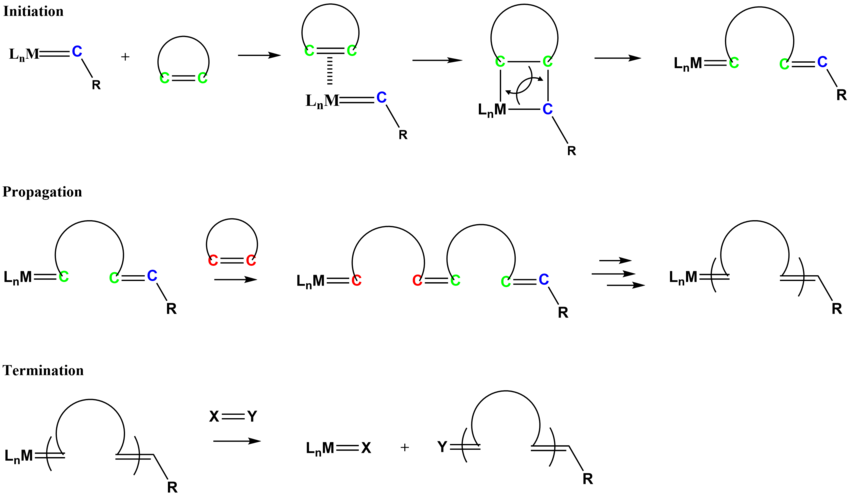
Commercially relevant unsaturated polymers synthesized by ROMP include polynorbornene, polycyclooctene, and polycyclopentadiene.[25]
Thermodynamics
[edit]The formal thermodynamic criterion of a given monomer polymerizability is related to a sign of the free enthalpy (Gibbs free energy) of polymerization: where:
- x and y indicate monomer and polymer states, respectively (x and/or y = l (liquid), g (gaseous), c (amorphous solid), c' (crystalline solid), s (solution));
- ΔHp(xy) is the enthalpy of polymerization (SI unit: joule per kelvin);
- ΔSp(xy) is the entropy of polymerization (SI unit: joule);
- T is the absolute temperature (SI unit: kelvin).
The free enthalpy of polymerization (ΔGp) may be expressed as a sum of standard enthalpy of polymerization (ΔGp°) and a term related to instantaneous monomer molecules and growing macromolecules concentrations: where:
- R is the gas constant;
- M is the monomer;
- (m)i is the monomer in an initial state;
- m* is the active monomer.
Following Flory–Huggins solution theory that the reactivity of an active center, located at a macromolecule of a sufficiently long macromolecular chain, does not depend on its degree of polymerization (DPi), and taking in to account that ΔGp° = ΔHp° − TΔSp° (where ΔHp° and ΔSp° indicate a standard polymerization enthalpy and entropy, respectively), we obtain:
At equilibrium (ΔGp = 0), when polymerization is complete the monomer concentration ([M]eq) assumes a value determined by standard polymerization parameters (ΔHp° and ΔSp°) and polymerization temperature: Polymerization is possible only when [M]0 > [M]eq. Eventually, at or above the so-called ceiling temperature (Tc), at which [M]eq = [M]0, formation of the high polymer does not occur. For example, tetrahydrofuran (THF) cannot be polymerized above Tc = 84 °C, nor cyclo-octasulfur (S8) below Tf = 159 °C.[26][27][28][29] However, for many monomers, Tc and Tf, for polymerization in the bulk, are well above or below the operable polymerization temperatures, respectively. The polymerization of a majority of monomers is accompanied by an entropy decrease, due mostly to the loss in the translational degrees of freedom. In this situation, polymerization is thermodynamically allowed only when the enthalpic contribution into ΔGp prevails (thus, when ΔHp° < 0 and ΔSp° < 0, the inequality |ΔHp| > −TΔSp is required). Therefore, the higher the ring strain, the lower the resulting monomer concentration at equilibrium.
Additional reading
[edit]- Luck, Russel M.; Sadhir, Rajender K., eds. (1992). Expanding Monomers: Synthesis, Characterization, and Applications. Boca Raton, Florida: CRC Press. ISBN 978-0-8493-5156-3.
- Nahrain E. Kamber; Wonhee Jeong; Robert M. Waymouth; Russell C. Pratt; Bas G. G. Lohmeijer; James L. Hedrick (2007). "Organocatalytic Ring-Opening Polymerization". Chemical Reviews. 107 (12): 5813–5840. doi:10.1021/cr068415b. PMID 17988157.
- Dubois, Philippe; Coulembier, Olivier; Raquez, Jean-Marie, eds. (2009). Handbook of Ring‐Opening Polymerization. Wiley. doi:10.1002/9783527628407. ISBN 9783527628407.
References
[edit]- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Ring-opening polymerization". doi:10.1351/goldbook.R05396
- ^ Jenkins, A. D.; Kratochvíl, P.; Stepto, R. F. T.; Suter, U. W. (1996). "Glossary of basic terms in polymer science (IUPAC Recommendations 1996)". Pure and Applied Chemistry. 68 (12): 2287–2311. doi:10.1351/pac199668122287.
- ^ Young, Robert J. (2011). Introduction to Polymers. Boca Raton: CRC Press. ISBN 978-0-8493-3929-5.
- ^ . doi:10.1007/s00726-006-0432-9.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ Nuyken, Oskar; Pask, Stephen (2013). "Ring-Opening Polymerization—An Introductory Review". Polymers. 5 (2): 361–403. doi:10.3390/polym5020361.
- ^ a b Yann Sarazin; Jean-François Carpentier (2015). "Discrete Cationic Complexes for Ring-Opening Polymerization Catalysis of Cyclic Esters and Epoxides". Chemical Reviews. 115 (9): 3564–3614. doi:10.1021/acs.chemrev.5b00033. PMID 25897976.
- ^ a b Longo, Julie M.; Sanford, Maria J.; Coates, Geoffrey W. (2016). "Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides with Discrete Metal Complexes: Structure–Property Relationships". Chemical Reviews. 116 (24): 15167–15197. doi:10.1021/acs.chemrev.6b00553. PMID 27936619.
- ^ a b JEROME, C; LECOMTE, P (2008-06-10). "Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization☆". Advanced Drug Delivery Reviews. 60 (9): 1056–1076. doi:10.1016/j.addr.2008.02.008. hdl:2268/3723. ISSN 0169-409X. PMID 18403043.
- ^ Matsumura, Shuichi; Tsukada, Keisuke; Toshima, Kazunobu (May 1997). "Enzyme-Catalyzed Ring-Opening Polymerization of 1,3-Dioxan-2-one to Poly(trimethylene carbonate)". Macromolecules. 30 (10): 3122–3124. Bibcode:1997MaMol..30.3122M. doi:10.1021/ma961862g.
- ^ Kricheldorf, H. R. (2006). "Polypeptides and 100 Years of Chemistry of α-Amino Acid N-Carboxyanhydrides". Angewandte Chemie International Edition. 45 (35): 5752–5784. doi:10.1002/anie.200600693. PMID 16948174.
- ^ Nikos Hadjichristidis; Hermis Iatrou; Marinos Pitsikalis; Georgios Sakellariou (2009). "Synthesis of Well-Defined Polypeptide-Based Materials via the Ring-Opening Polymerization of α-Amino Acid N-Carboxyanhydrides". Chemical Reviews. 109 (11): 5528–5578. doi:10.1021/cr900049t. PMID 19691359.
- ^ Scott, R. J.; Gunning, H. E. (1952). "The Polymerization of Cyclopropane". J. Phys. Chem. 56 (1): 156–160. doi:10.1021/j150493a031.
- ^ Yokozawa, Tsutomu; Tsuruta, Ei-ichi (1996). "Ring-Opening Polymerization of the Cyclobutane Adduct of Methyl Tricyanoethylenecarboxylate and Ethyl Vinyl Ether". Macromolecules. 29 (25): 8053–8056. doi:10.1021/ma9608535.
- ^ Leuchs, H. (1906). "Glycine-carbonic acid". Berichte der Deutschen Chemischen Gesellschaft. 39: 857. doi:10.1002/cber.190603901133.
- ^ Dainton, F. S.; Devlin, T. R. E.; Small, P. A. (1955). "The thermodynamics of polymerization of cyclic compounds by ring opening". Transactions of the Faraday Society. 51: 1710. doi:10.1039/TF9555101710.
- ^ Conix, André; Smets, G. (January 1955). "Ring opening in lactam polymers". Journal of Polymer Science. 15 (79): 221–229. Bibcode:1955JPoSc..15..221C. doi:10.1002/pol.1955.120157918.
- ^ Kałuz̀ynski, Krzysztof; Libiszowski, Jan; Penczek, Stanisław (1977). "Poly(2-hydro-2-oxo-1,3,2-dioxaphosphorinane). Preparation and NMR spectra". Die Makromolekulare Chemie. 178 (10): 2943–2947. doi:10.1002/macp.1977.021781017. ISSN 0025-116X.
- ^ Libiszowski, Jan; Kałużynski, Krzysztof; Penczek, Stanisław (June 1978). "Polymerization of cyclic esters of phosphoric acid. VI. Poly(alkyl ethylene phosphates). Polymerization of 2-alkoxy-2-oxo-1,3,2-dioxaphospholans and structure of polymers". Journal of Polymer Science: Polymer Chemistry Edition. 16 (6): 1275–1283. Bibcode:1978JPoSA..16.1275L. doi:10.1002/pol.1978.170160610.
- ^ a b c d e f Nuyken, Oskar; Stephen D. Pask (25 April 2013). "Ring-Opening Polymerization—An Introductory Review". Polymers. 5 (2): 361–403. doi:10.3390/polym5020361.
- ^ a b c d e Dubois, Philippe (2008). Handbook of ring-opening polymerization (1. Aufl. ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-31953-4.
- ^ Cowie, John McKenzie Grant (2008). Polymers: Chemistry and Physics of Modern Materials. Boca Raton, Florida: CRC Press. pp. 105–107. ISBN 978-0-8493-9813-1.
- ^ Pruckmayr, Gerfried; Dreyfuss, P.; Dreyfuss, M. P. (1996). "Polyethers, Tetrahydrofuran and Oxetane Polymers". Kirk‑Othmer Encyclopedia of Chemical Technology. John Wiley & Sons.
- ^ Sutthasupa, Sutthira; Shiotsuki, Masashi; Sanda, Fumio (13 October 2010). "Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials". Polymer Journal. 42 (12): 905–915. doi:10.1038/pj.2010.94.
- ^ Hartwig, John F. (2010). Organotransition metal chemistry: from bonding to catalysis. Sausalito, California: University Science Books. ISBN 978-1-891389-53-5.
- ^ Walsh, Dylan J.; Lau, Sii Hong; Hyatt, Michael G.; Guironnet, Damien (2017-09-25). "Kinetic Study of Living Ring-Opening Metathesis Polymerization with Third-Generation Grubbs Catalysts". Journal of the American Chemical Society. 139 (39): 13644–13647. doi:10.1021/jacs.7b08010. ISSN 0002-7863. PMID 28944665.
- ^ Tobolsky, A. V. (July 1957). "Equilibrium polymerization in the presence of an ionic initiator". Journal of Polymer Science. 25 (109): 220–221. Bibcode:1957JPoSc..25..220T. doi:10.1002/pol.1957.1202510909.
- ^ Tobolsky, A. V. (August 1958). "Equilibrium polymerization in the presence of an ionic initiator". Journal of Polymer Science. 31 (122): 126. Bibcode:1958JPoSc..31..126T. doi:10.1002/pol.1958.1203112214.
- ^ Tobolsky, Arthur V.; Eisenberg, Adi (May 1959). "Equilibrium Polymerization of Sulfur". Journal of the American Chemical Society. 81 (4): 780–782. doi:10.1021/ja01513a004.
- ^ Tobolsky, A. V.; Eisenberg, A. (January 1960). "A General Treatment of Equilibrium Polymerization". Journal of the American Chemical Society. 82 (2): 289–293. doi:10.1021/ja01487a009.


![{\displaystyle \Delta G_{p}=\Delta G_{p}^{\circ }+RT\ln {\frac {[\ldots -({\ce {m}})_{i+1}{\ce {m}}^{\ast }]}{[{\ce {M}}][\ldots -({\ce {m}})_{i}{\ce {m}}^{\ast }]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4b2f97536afb3600033e87d3cbcf35aebb153c7e)
![{\displaystyle \Delta G_{p}=\Delta H_{p}^{\circ }-T(\Delta S_{p}^{\circ }+R\ln[M])}](https://wikimedia.org/api/rest_v1/media/math/render/svg/220a922430e9e4f58818db0e2be969135dd4db24)
![{\displaystyle {\begin{aligned}{}[{\ce {M}}]_{\rm {eq}}&=\exp \left({\frac {\Delta H_{p}^{\circ }}{RT}}-{\frac {\Delta S_{p}^{\circ }}{R}}\right)\\[4pt]\ln {\frac {DP_{n}}{DP_{n}-1}}[{\ce {M}}]_{\rm {eq}}&={\frac {\Delta H_{p}^{\circ }}{RT}}-{\frac {\Delta S_{p}^{\circ }}{R}}\\[4pt][{\ce {M}}]_{\rm {eq}}&={\frac {DP_{n}-1}{DP_{n}}}\exp \left({\frac {\Delta H_{p}^{\circ }}{RT}}-{\frac {\Delta S_{p}^{\circ }}{R}}\right)\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1ea199b956f752a32271601e036e6736a8c8c50d)
![{\displaystyle {\begin{aligned}T_{c}&={\frac {\Delta H_{p}^{\circ }}{\Delta S_{p}^{\circ }+R\ln[{\ce {M}}]_{0}}};\quad (\Delta H_{p}^{\circ }<0,\ \Delta S_{p}^{\circ }<0)\\[4pt]T_{f}&={\frac {\Delta H_{p}^{\circ }}{\Delta S_{p}^{\circ }+R\ln[{\ce {M}}]_{0}}};\quad (\Delta H_{p}^{\circ }>0,\ \Delta S_{p}^{\circ }>0)\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3e255372d1a8b146c1a15fe68d7b394f54664f79)