RNA world
The RNA world is a hypothetical stage in the evolutionary history of life on Earth in which self-replicating RNA molecules proliferated before the evolution of DNA and proteins.[1] The term also refers to the hypothesis that posits the existence of this stage. Alexander Rich first proposed the concept of the RNA world in 1962,[2] and Walter Gilbert coined the term in 1986.[3]
Among the characteristics of RNA that suggest its original prominence are that:
- Like DNA, RNA can store and replicate genetic information. Although RNA is considerably more fragile than DNA, some ancient RNAs may have evolved the ability to methylate other RNAs to protect them.[4] The concurrent formation of all four RNA building blocks further strengthens the hypothesis.[5]
- Enzymes made of RNA (ribozymes) can catalyze (start or accelerate) chemical reactions that are critical for life,[6] so it is conceivable that in an RNA world, ribozymes might have preceded enzymes made of protein.
- Many coenzymes that have fundamental roles in cellular life, such as acetyl-CoA, NADH, FADH, and F420, are structurally strikingly similar to RNA and so may be surviving remnants of covalently bound coenzymes in an RNA world.[7]
- One of the most critical components of cells, the ribosome, is composed primarily of RNA.
Although alternative chemical paths to life have been proposed,[8] and RNA-based life may not have been the first life to exist, [3][9] the RNA world hypothesis seems to be the most favored abiogenesis paradigm. However, even proponents agree that there is still has not conclusive evidence to completely falsify other paradigms and hypotheses.[2][10][11] Regardless of its plausibility in a prebiotic scenario, the RNA world can serve as a model system for studying the origin of life.[12]
If the RNA world existed, it was probably followed by an age characterized by the evolution of ribonucleoproteins (RNP world),[3] which in turn ushered in the era of DNA and longer proteins. DNA has greater stability and durability than RNA, which may explain why it became the predominant information storage molecule.[13] Protein enzymes may have replaced RNA-based ribozymes as biocatalysts because the greater abundance and diversity of the monomers of which they are built makes them more versatile. As some cofactors contain both nucleotide and amino-acid characteristics, it may be that amino acids, peptides, and finally proteins initially were cofactors for ribozymes.[7]
History
[edit]One of the challenges in studying abiogenesis is that the system of reproduction and metabolism utilized by all extant life involves three distinct types of interdependent macromolecules (DNA, RNA, and proteins). This suggests that life could not have arisen in its current form, which has led researchers to hypothesize mechanisms whereby the current system might have arisen from a simpler precursor system.[14] American molecular biologist Alexander Rich was the first to posit a coherent hypothesis on the origin of nucleotides as precursors of life.[15] In an article he contributed to a volume issued in honor of Nobel-laureate physiologist Albert Szent-Györgyi, he explained that the primitive Earth's environment could have produced RNA molecules (polynucleotide monomers) that eventually acquired enzymatic and self-replicating functions.[16]
Other mentions of RNA as a primordial molecule can be found in papers by Francis Crick[17] and Leslie Orgel,[18] as well as in Carl Woese's 1967 book The Genetic Code.[19] Hans Kuhn in 1972 laid out a possible process by which the modern genetic system might have arisen from a nucleotide-based precursor, and this led Harold White in 1976 to observe that many of the cofactors essential for enzymatic function are either nucleotides or could have been derived from nucleotides. He proposed a scenario whereby the critical electrochemistry of enzymatic reactions would have necessitated retention of the specific nucleotide moieties of the original RNA-based enzymes carrying out the reactions, while the remaining structural elements of the enzymes were gradually replaced by protein, until all that remained of the original RNAs were these nucleotide cofactors, "fossils of nucleic acid enzymes".[20]
Properties of RNA
[edit]The properties of RNA make the idea of the RNA world hypothesis conceptually plausible, though its general acceptance as an explanation for the origin of life requires further evidence.[21] RNA is known to form efficient catalysts, and its similarity to DNA makes clear its ability to store information. Opinions differ, however, as to whether RNA constituted the first autonomous self-replicating system or was a derivative of a still-earlier system.[3] One version of the hypothesis is that a different type of nucleic acid, termed pre-RNA, was the first one to emerge as a self-reproducing molecule, to be replaced by RNA only later. On the other hand, the discovery in 2009 that activated pyrimidine ribonucleotides can be synthesized under plausible prebiotic conditions[22] suggests that it is premature to dismiss the RNA-first scenarios.[3] Suggestions for 'simple' pre-RNA nucleic acids have included peptide nucleic acid (PNA), threose nucleic acid (TNA) or glycol nucleic acid (GNA).[23][24] Despite their structural simplicity and possession of properties comparable with RNA, the chemically plausible generation of "simpler" nucleic acids under prebiotic conditions has yet to be demonstrated.[25]
RNA as an enzyme
[edit]In the 1980s, RNA structures capable of self-processing were discovered,[26] with the RNA moiety of RNase P acting as its catalytic subunit.[27] These catalytic RNAs were referred to as RNA enzymes, or ribozymes, are found in today's DNA-based life and could be examples of living fossils. Ribozymes play vital roles, such as that of the ribosome. The large subunit of the ribosome includes an rRNA responsible for the peptide bond-forming peptidyl transferase activity of protein synthesis. Many other ribozyme activities exist; for example, the hammerhead ribozyme performs self-cleavage[28] and an RNA polymerase ribozyme can synthesize a short RNA strand from a primed RNA template.[29]
Among the enzymatic properties important for the beginning of life are:
- Self-replication
- The ability to self-replicate or synthesize other RNA molecules; relatively short RNA molecules that can synthesize others have been artificially produced in the lab. The shortest was 165 bases long, though it has been estimated that only part of the molecule was crucial for this function. One version, 189 bases long, had an error rate of just 1.1% per nucleotide when synthesizing an 11-nucleotide long RNA strand from primed template strands.[30] This 189-base pair ribozyme could polymerize a template of at most 14 nucleotides in length, which is too short for self-replication, but is a potential lead for further investigation. The longest primer extension performed by a ribozyme polymerase was 20 bases.[31] In 2016, researchers reported the use of in vitro evolution to improve dramatically the activity and generality of an RNA polymerase ribozyme by selecting variants that can synthesize functional RNA molecules from an RNA template.[32] Each RNA polymerase ribozyme was engineered to remain linked to its new, synthesized RNA strand; this allowed the team to isolate successful polymerases. The isolated RNA polymerases were again used for another round of evolution. After several rounds of evolution, they obtained one RNA polymerase ribozyme called 24-3 that was able to copy almost any other RNA, from small catalysts to long RNA-based enzymes. Particular RNAs were amplified up to 10,000 times, a first RNA version of the polymerase chain reaction (PCR).[32]
- Catalysis
- The ability to catalyze simple chemical reactions—which would enhance creation of molecules that are building blocks of RNA molecules (i.e., a strand of RNA that would make creating more strands of RNA easier). Relatively short RNA molecules with such abilities have been artificially formed in the lab.[33][34] A recent study showed that almost any nucleic acid can evolve into a catalytic sequence under appropriate selection. For instance, an arbitrarily chosen 50-nucleotide DNA fragment encoding for the Bos taurus (cattle) albumin mRNA was subjected to test-tube evolution to derive a catalytic DNA (Deoxyribozyme, also called DNAzyme) with RNA-cleavage activity. After only a few weeks, a DNAzyme with significant catalytic activity had evolved.[35] In general, DNA is much more chemically inert than RNA and hence much more resistant to obtaining catalytic properties. If in vitro evolution works for DNA it will happen much more easily with RNA. In 2022, Nick Lane and coauthors showed in a computational simulation that short RNA sequences could have been capable of catalyzing CO2 fixation which supported protocell replication and growth.[36]
- Amino acid-RNA ligation
- The ability to conjugate an amino acid to the 3'-end of an RNA in order to use its chemical groups or provide a long-branched aliphatic sidechain.[37]
- Peptide bond formation
- The ability to catalyse the formation of peptide bonds between amino acids to produce short peptides or longer proteins. This is done in modern cells by ribosomes, a complex of several RNA molecules known as rRNA together with many proteins. The rRNA molecules are thought responsible for its enzymatic activity, as no amino-acid residues lie within 18Å of the enzyme's active site,[21] and, when the majority of the amino-acid residues in the ribosome were stringently removed, the resulting ribosome retained its full peptidyl transferase activity, fully able to catalyze the formation of peptide bonds between amino acids.[38] A pseudo 2 fold symmetry of the region surrounding the peptidyl transferase center led to the hypothesis of the Proto-Ribosome, that a vestige of an ancient dimeric molecule from the RNA world is functioning within the ribosome.[39] An RNA molecule with the ribosomal RNA sequence has been synthesized in the lab to test the Proto-ribosome hypothesis and was able to dimerize and to form peptide bonds.[40] A much shorter RNA molecule has been synthesized in the laboratory with the ability to form peptide bonds, and it has been suggested that rRNA has evolved from a similar molecule.[41] It has also been suggested that amino acids may have initially been involved with RNA molecules as cofactors enhancing or diversifying their enzymatic capabilities, before evolving into more complex peptides. Similarly, tRNA is suggested to have evolved from RNA molecules that began to catalyze amino acid transfer.[42]
Cofactors
[edit]- Protein enzymes catalyze various chemical reactions, but over half of them incorporate cofactors to facilitate and diversify their catalytic activities.[43] Cofactors are essential in biology, as they are based largely on nucleotides rather than amino acids. Ribozymes use nucleotide cofactors to create metabolism, with two basic choices: non-covalent binding or covalent attachment. Both approaches have been demonstrated using directed evolution to reinvent RNA dupes of protein-catalyzed processes. Lorsch and Szostak [44] investigated ribozymes that could phosphorylate themselves and use ATP-γS as a substrate. However, only one of the seven classes of selected ribozymes had detectable ATP affinity, indicating that the ability to bind ATP was compromised. NAD+- dependent redox ribozymes were also evaluated.[45] The select ribozyme had a rate of enhancement of more than 107 fold and was proven to catalyze the reverse reaction - benzaldehyde reduction by NADH.[46] Since the usage of adenosine as a cofactor is prevalent in current metabolism and is likely to have been common in the RNA world, these discoveries are essential for the evolution of metabolism in the RNA world.
RNA in information storage
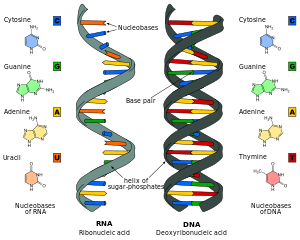
[edit]RNA is a very similar molecule to DNA, with only two significant chemical differences (the backbone of RNA uses ribose instead of deoxyribose and its nucleobases include uracil instead of thymine). The overall structure of RNA and DNA are immensely similar—one strand of DNA and one of RNA can bind to form a double helical structure. This makes the storage of information in RNA possible in a very similar way to the storage of information in DNA. However, RNA is less stable, being more prone to hydrolysis due to the presence of a hydroxyl group at the ribose 2' position.

Comparison of DNA and RNA structure
[edit]The major difference between RNA and DNA is the presence of a hydroxyl group at the 2'-position of the ribose sugar in RNA (illustration, right).[21] This group makes the molecule less stable because, when not constrained in a double helix, the 2' hydroxyl can chemically attack the adjacent phosphodiester bond to cleave the phosphodiester backbone. The hydroxyl group also forces the ribose into the C3'-endo sugar conformation unlike the C2'-endo conformation of the deoxyribose sugar in DNA. This forces an RNA double helix to change from a B-DNA structure to one more closely resembling A-DNA.
RNA also uses a different set of bases than DNA—adenine, guanine, cytosine and uracil, instead of adenine, guanine, cytosine and thymine. Chemically, uracil is similar to thymine, differing only by a methyl group, and its production requires less energy.[47] In terms of base pairing, this has no effect. Adenine readily binds uracil or thymine. Uracil is, however, one product of damage to cytosine that makes RNA particularly susceptible to mutations that can replace a GC base pair with a GU (wobble) or AU base pair.
RNA is thought to have preceded DNA, because of their ordering in the biosynthetic pathways.[9] The deoxyribonucleotides used to make DNA are made from ribonucleotides, the building blocks of RNA, by removing the 2'-hydroxyl group. As a consequence, a cell must have the ability to make RNA before it can make DNA.
Limitations of information storage in RNA
[edit]The chemical properties of RNA make large RNA molecules inherently fragile, and they can easily be broken down into their constituent nucleotides through hydrolysis.[48][49] These limitations do not make use of RNA as an information storage system impossible, simply energy intensive (to repair or replace damaged RNA molecules) and prone to mutation. While this makes it unsuitable for current 'DNA optimised' life, it may have been acceptable for more primitive life.
RNA as a regulator
[edit]Riboswitches have been found to act as regulators of gene expression, particularly in bacteria, but also in plants and archaea. Riboswitches alter their secondary structure in response to the binding of a metabolite. Riboswitch classes have highly conserved aptamer domains, even among diverse organisms. When a target metabolite is bound to this aptamer, conformational changes occur, modulating the expression of genes carried by mRNA. These changes occur in an expression platform, located downstream from the aptamer.[50] This change in structure can result in the formation or disruption of a terminator, truncating or permitting transcription respectively.[51] Alternatively, riboswitches may bind or occlude the Shine–Dalgarno sequence, affecting translation.[52] It has been suggested that these originated in an RNA-based world.[53] In addition, RNA thermometers regulate gene expression in response to temperature changes.[54]
Support and difficulties
[edit]The RNA world hypothesis is supported by RNA's ability to do all three of to store, to transmit, and to duplicate genetic information, as DNA does, and to perform enzymatic reactions, like protein-based enzymes. Because it can carry out the types of tasks now performed by proteins and DNA, RNA is believed to have once been capable of supporting independent life on its own.[21] Some viruses use RNA as their genetic material, rather than DNA.[55] Further, while nucleotides were not found in experiments based on Miller-Urey experiment, their formation in prebiotically plausible conditions was reported in 2009;[22] a purine base, adenine, is merely a pentamer of hydrogen cyanide, and it happens that this particular base is used as omnipresent energy vehicle in the cell: adenosine triphosphate is used everywhere in preference to guanosine triphosphate, cytidine triphosphate, uridine triphosphate or even deoxythymidine triphosphate, which could serve just as well but are practically never used except as building blocks for nucleic acid chains. Experiments with basic ribozymes, like Bacteriophage Qβ RNA, have shown that simple self-replicating RNA structures can withstand even strong selective pressures (e.g., opposite-chirality chain terminators).[56]
Since there were no known chemical pathways for the abiogenic synthesis of nucleotides from pyrimidine nucleobases cytosine and uracil under prebiotic conditions, it is thought by some that nucleic acids did not contain these nucleobases seen in life's nucleic acids.[57] The nucleoside cytosine has a half-life in isolation of 19 days at 100 °C (212 °F) and 17,000 years in freezing water, which some argue is too short on the geologic time scale for accumulation.[58] Others have questioned whether ribose and other backbone sugars could be stable enough to be found in the original genetic material,[59] and have raised the issue that all ribose molecules would have had to be the same enantiomer, as any nucleotide of the wrong chirality acts as a chain terminator.[60]
Pyrimidine ribonucleosides and their respective nucleotides have been prebiotically synthesised by a sequence of reactions that by-pass free sugars and assemble in a stepwise fashion by including nitrogenous and oxygenous chemistries. In a series of publications, John Sutherland and his team at the School of Chemistry, University of Manchester, have demonstrated high yielding routes to cytidine and uridine ribonucleotides built from small 2- and 3-carbon fragments such as glycolaldehyde, glyceraldehyde or glyceraldehyde-3-phosphate, cyanamide, and cyanoacetylene. One of the steps in this sequence allows the isolation of enantiopure ribose aminooxazoline if the enantiomeric excess of glyceraldehyde is 60% or greater, of possible interest toward biological homochirality.[61] This can be viewed as a prebiotic purification step, where the said compound spontaneously crystallised out from a mixture of the other pentose aminooxazolines. Aminooxazolines can react with cyanoacetylene in a mild and highly efficient manner, controlled by inorganic phosphate, to give the cytidine ribonucleotides. Photoanomerization with UV light allows for inversion about the 1' anomeric centre to give the correct beta stereochemistry; one problem with this chemistry is the selective phosphorylation of alpha-cytidine at the 2' position.[62] However, in 2009, they showed that the same simple building blocks allow access, via phosphate controlled nucleobase elaboration, to 2',3'-cyclic pyrimidine nucleotides directly, which are known to be able to polymerise into RNA.[22] Organic chemist Donna Blackmond described this finding as "strong evidence" in favour of the RNA world.[63] However, John Sutherland said that while his team's work suggests that nucleic acids played an early and central role in the origin of life, it did not necessarily support the RNA world hypothesis in the strict sense, which he described as a "restrictive, hypothetical arrangement".[64]
The Sutherland group's 2009 paper also highlighted the possibility for the photo-sanitization of the pyrimidine-2',3'-cyclic phosphates.[22] A potential weakness of these routes is the generation of enantioenriched glyceraldehyde, or its 3-phosphate derivative (glyceraldehyde prefers to exist as its keto tautomer dihydroxyacetone).[citation needed]
On August 8, 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting building blocks of RNA (adenine, guanine, and related organic molecules) may have been formed in outer space.[65][66][67] In 2017, research using a numerical model suggested that a RNA world may have emerged in warm ponds on the early Earth, and that meteorites were a plausible and probable source of the RNA building blocks (ribose and nucleic acids) to these environments.[68] On August 29, 2012, astronomers at Copenhagen University reported the detection of a specific sugar molecule, glycolaldehyde, in a distant star system. The molecule was found around the protostellar binary IRAS 16293-2422, which is located 400 light years from Earth.[69][70] Because glycolaldehyde is needed to form RNA, this finding suggests that complex organic molecules may form in stellar systems prior to the formation of planets, eventually arriving on young planets early in their formation.[71] Nitriles, key molecular precursors of the RNA World scenario, are among the most abundant chemical families in the universe and have been found in molecular clouds in the center of the Milky Way, protostars of different masses, meteorites and comets, and also in the atmosphere of Titan, the largest moon of Saturn.[72][73]
A study in 2001 shows that nicotinic acid and its precursor, quinolinic acid can be "produced in yields as high as 7% in a six-step nonenzymatic sequence from aspartic acid and dihydroxyacetone phosphate (DHAP). The biosynthesis of ribose phosphate could have produced DHAP and other three carbon compounds. Aspartic acid could have been available from prebiotic synthesis or from the ribozyme synthesis of pyrimidines." This supports that NAD could have originated in the RNA world.[74] RNA sequences at lengths of 30 nucleotides, 60 nucleotides, 100 nucleotides, and 140 nucleotides, were capable of catalysis of "the synthesis of three common coenzymes, CoA, NAD, and FAD, from their precursors, 4‘-phosphopantetheine, NMN, and FMN, respectively".[75]
Prebiotic RNA synthesis
[edit]
Nucleotides are the fundamental molecules that combine in series to form RNA. They consist of a nitrogenous base attached to a sugar-phosphate backbone. RNA is made of long stretches of specific nucleotides arranged so that their sequence of bases carries information. The RNA world hypothesis holds that in the primordial soup (or sandwich), there existed free-floating nucleotides. These nucleotides regularly formed bonds with one another, which often broke because the change in energy was so low. However, certain sequences of base pairs have catalytic properties that lower the energy of their chain being created, enabling them to stay together for longer periods of time. As each chain grew longer, it attracted more matching nucleotides faster, causing chains to now form faster than they were breaking down.
These chains have been proposed by some as the first, primitive forms of life. In an RNA world, different sets of RNA strands would have had different replication outputs, which would have increased or decreased their frequency in the population, i.e., natural selection. As the fittest sets of RNA molecules expanded their numbers, novel catalytic properties added by mutation, which benefitted their persistence and expansion, could accumulate in the population. Such an autocatalytic set of ribozymes, capable of self-replication in about an hour, has been identified. It was produced by molecular competition (in vitro evolution) of candidate enzyme mixtures.[76]
Competition between RNA may have favored the emergence of cooperation between different RNA chains, opening the way for the formation of the first protocell. Eventually, RNA chains developed with catalytic properties that help amino acids bind together (a process called peptide-bonding). These amino acids could then assist with RNA synthesis, giving those RNA chains that could serve as ribozymes the selective advantage. The ability to catalyze one step in protein synthesis, aminoacylation of RNA, has been demonstrated in a short (five-nucleotide) segment of RNA.[77]
In March 2015, NASA scientists reported that, for the first time, complex DNA and RNA organic compounds of life, including uracil, cytosine, and thymine, have been formed in the laboratory under conditions found only in outer space, using starting chemicals, like pyrimidine, found in meteorites. Pyrimidine, like polycyclic aromatic hydrocarbons (PAHs), may have been formed in red giant stars or in interstellar dust and gas clouds, according to the scientists.[78]
In 2018, researchers at Georgia Institute of Technology identified three molecular candidates for the bases that might have formed an earliest version of proto-RNA: barbituric acid, melamine, and 2,4,6-triaminopyrimidine (TAP). These three molecules are simpler versions of the four bases in current RNA, which could have been present in larger amounts and could still be forward-compatible with them but may have been discarded by evolution in exchange for more optimal base pairs.[79] Specifically, TAP can form nucleotides with a large range of sugars.[80] Both TAP and melamine base pair with barbituric acid. All three spontaneously form nucleotides with ribose.[81]
Evolution of DNA
[edit]One of the challenges posed by the RNA world hypothesis is to discover the pathway by which an RNA-based system transitioned to one based on DNA. Geoffrey Diemer and Ken Stedman, at Portland State University in Oregon, may have found a solution. While conducting a survey of viruses in a hot acidic lake in Lassen Volcanic National Park, California, they uncovered evidence that a simple DNA virus had acquired a gene from a completely unrelated RNA-based virus. Virologist Luis Villareal of the University of California Irvine also suggests that viruses capable of converting an RNA-based gene into DNA and then incorporating it into a more complex DNA-based genome might have been common in the virus world during the RNA to DNA transition some 4 billion years ago.[82][83] This finding bolsters the argument for the transfer of information from the RNA world to the emerging DNA world before the emergence of the last universal common ancestor. From the research, the diversity of this virus world is still with us.
Viroids
[edit]Additional evidence supporting the concept of an RNA world has resulted from research on viroids, the first representatives of a novel domain of "subviral pathogens".[84][85] Viroids infect plants, where most are pathogens, and consist of short stretches of highly complementary, circular, single-stranded and non-coding RNA without a protein coat. They are extremely small, ranging from 246 to 467 nucleobases, compared to the smallest known viruses capable of causing an infection, with genomes about 2,000 nucleobases in length.[86]
Based on their characteristic properties, in 1989 plant biologist Theodor Diener argued that viroids are more plausible living relics of the RNA world than introns and other RNAs considered candidates at the time.[87] Diener's hypothesis would be expanded by the research group of Ricardo Flores,[88][89] and gained a broader audience when in 2014, a New York Times science writer published a popularized version of the proposal.[90]
The characteristics of viroids highlighted as consistent with an RNA world were their small size, high guanine and cytosine content, circular structure, structural periodicity, the lack of protein-coding ability and, in some cases, ribozyme-mediated replication.[89] One aspect critics of the hypothesis have focused on is that the exclusive hosts of all known viroids, angiosperms, did not evolve until billions of years after the RNA world was replaced, making viroids more likely to have arisen through later evolutionary mechanisms unrelated to the RNA world than to have survived via a cryptic host over that extended period.[91] Whether they are relics of that world or of more recent origin, their function as autonomous naked RNA is seen as analogous to that envisioned for an RNA world.
Origin of sexual reproduction
[edit]Eigen et al.[92] and Woese[93] proposed that the genomes of early protocells were composed of single-stranded RNA, and that individual genes corresponded to separate RNA segments, rather than being linked end-to-end as in present-day DNA genomes. A protocell that was haploid (one copy of each RNA gene) would be vulnerable to damage, since a single lesion in any RNA segment would be potentially lethal to the protocell (e.g., by blocking replication or inhibiting the function of an essential gene).
Vulnerability to damage could be reduced by maintaining two or more copies of each RNA segment in each protocell, i.e., by maintaining diploidy or polyploidy. Genome redundancy would allow a damaged RNA segment to be replaced by an additional replication of its homolog. However, for such a simple organism, the proportion of available resources tied up in the genetic material would be a large fraction of the total resource budget. Under limited resource conditions, the protocell reproductive rate would likely be inversely related to ploidy number. The protocell's fitness would be reduced by the costs of redundancy. Consequently, coping with damaged RNA genes while minimizing the costs of redundancy would likely have been a fundamental problem for early protocells.
A cost-benefit analysis was carried out in which the costs of maintaining redundancy were balanced against the costs of genome damage.[94] This analysis led to the conclusion that, under a wide range of circumstances, the selected strategy would be for each protocell to be haploid, but to periodically fuse with another haploid protocell to form a transient diploid. The retention of the haploid state maximizes the growth rate. The periodic fusions permit mutual reactivation of otherwise lethally damaged protocells. If at least one damage-free copy of each RNA gene is present in the transient diploid, viable progeny can be formed. For two, rather than one, viable daughter cells to be produced would require an extra replication of the intact RNA gene homologous to any RNA gene that had been damaged prior to the division of the fused protocell. The cycle of haploid reproduction, with occasional fusion to a transient diploid state, followed by splitting to the haploid state, can be considered to be the sexual cycle in its most primitive form.[94][95] In the absence of this sexual cycle, haploid protocells with damage in an essential RNA gene would simply die.
This model for the early sexual cycle is hypothetical, but it is very similar to the known sexual behavior of the segmented RNA viruses, which are among the simplest organisms known. Influenza virus, whose genome consists of 8 physically separated single-stranded RNA segments,[96] is an example of this type of virus. In segmented RNA viruses, "mating" can occur when a host cell is infected by at least two virus particles. If these viruses each contain an RNA segment with a lethal damage, multiple infection can lead to reactivation providing that at least one undamaged copy of each virus gene is present in the infected cell. This phenomenon is known as "multiplicity reactivation". Multiplicity reactivation has been reported to occur in influenza virus infections after induction of RNA damage by UV-irradiation,[97] and ionizing radiation.[98]
Further developments
[edit]Patrick Forterre has been working on a novel hypothesis, called "three viruses, three domains":[99] that viruses were instrumental in the transition from RNA to DNA and the evolution of Bacteria, Archaea, and Eukaryota. He believes the last universal common ancestor[99] was RNA-based and evolved RNA viruses. Some of the viruses evolved into DNA viruses to protect their genes from attack. Through the process of viral infection into hosts the three domains of life evolved.[99][100]
Another interesting proposal is the idea that RNA synthesis might have been driven by temperature gradients, in the process of thermosynthesis.[101] Single nucleotides have been shown to catalyze organic reactions.[102]
Steven Benner has argued that chemical conditions on the planet Mars, such as the presence of boron, molybdenum, and oxygen, may have been better for initially producing RNA molecules than those on Earth. If so, life-suitable molecules, originating on Mars, may have later migrated to Earth via mechanisms of panspermia or similar process.[103][104]
Alternative hypotheses
[edit]The hypothesized existence of an RNA world does not exclude a "Pre-RNA world", where a metabolic system based on a different nucleic acid is proposed to pre-date RNA. A candidate nucleic acid is peptide nucleic acid (PNA), which uses simple peptide bonds to link nucleobases.[105] PNA is more stable than RNA, but its ability to be generated under prebiological conditions has yet to be demonstrated experimentally.[citation needed]
Threose nucleic acid (TNA) or glycol nucleic acid (GNA) have also been proposed as a starting point, and like PNA, also lack experimental evidence for their respective abiogenesis.[citation needed]
An alternative—or complementary—theory of RNA origin is proposed in the PAH world hypothesis, whereby polycyclic aromatic hydrocarbons (PAHs) mediate the synthesis of RNA molecules.[106] PAHs are the most common and abundant of the known polyatomic molecules in the visible Universe and are a likely constituent of the primordial sea.[107] PAHs and fullerenes (also implicated in the origin of life)[108] have been detected in nebulae.[109]
The iron-sulfur world theory proposes that simple metabolic processes developed before genetic materials did, and these energy-producing cycles catalyzed the production of genes.[citation needed]
Some of the difficulties of producing the precursors on earth are bypassed by another alternative or complementary theory for their origin, panspermia. It discusses the possibility that the earliest life on this planet was carried here from somewhere else in the galaxy, possibly on meteorites similar to the Murchison meteorite.[110] Sugar molecules, including ribose, have been found in meteorites.[111][112] Panspermia does not invalidate the concept of an RNA world, but posits that this world or its precursors originated not on Earth but rather another, probably older, planet.[citation needed]
The relative chemical complexity of the nucleotide and the unlikelihood of it spontaneously arising, along with the limited number of combinations possible among four base forms, as well as the need for RNA polymers of some length before seeing enzymatic activity, have led some to reject the RNA world hypothesis in favor of a metabolism-first hypothesis, where the chemistry underlying cellular function arose first, along with the ability to replicate and facilitate this metabolism.[citation needed]
RNA-peptide coevolution
[edit]Another proposal is that the dual-molecule system we see today, where a nucleotide-based molecule is needed to synthesize protein, and a peptide-based (protein) molecule is needed to make nucleic acid polymers, represents the original form of life.[113] This theory is called RNA-peptide coevolution,[114] or the Peptide-RNA world, and offers a possible explanation for the rapid evolution of high-quality replication in RNA (since proteins are catalysts), with the disadvantage of having to postulate the coincident formation of two complex molecules, an enzyme (from peptides) and a RNA (from nucleotides). In this Peptide-RNA World scenario, RNA would have contained the instructions for life, while peptides (simple protein enzymes) would have accelerated key chemical reactions to carry out those instructions.[115] The study leaves open the question of exactly how those primitive systems managed to replicate themselves — something neither the RNA World hypothesis nor the Peptide-RNA World theory can yet explain, unless polymerases (enzymes that rapidly assemble the RNA molecule) played a role.[115]
A research project completed in March 2015 by the Sutherland group found that a network of reactions beginning with hydrogen cyanide and hydrogen sulfide, in streams of water irradiated by UV light, could produce the chemical components of proteins and lipids, alongside those of RNA.[116][117] The researchers used the term "cyanosulfidic" to describe this network of reactions.[116] In November 2017, a team at the Scripps Research Institute identified reactions involving the compound diamidophosphate which could have linked the chemical components into short peptide and lipid chains as well as short RNA-like chains of nucleotides.[118][119]
Implications
[edit]The RNA world hypothesis, if true, has important implications for the definition of life and the origin of life. For most of the time that followed Franklin, Watson and Crick's elucidation of DNA structure in 1953, life was largely defined in terms of DNA and proteins: DNA and proteins seemed the dominant macromolecules in the living cell, with RNA only aiding in creating proteins from the DNA blueprint.
The RNA world hypothesis places RNA at center-stage when life originated. The RNA world hypothesis is supported by the observations that ribosomes are ribozymes:[120][121] the catalytic site is composed of RNA, and proteins hold no major structural role and are of peripheral functional importance. This was confirmed with the deciphering of the 3-dimensional structure of the ribosome in 2001. Specifically, peptide bond formation, the reaction that binds amino acids together into proteins, is now known to be catalyzed by an adenine residue in the rRNA.
RNAs are known to play roles in other cellular catalytic processes, specifically in the targeting of enzymes to specific RNA sequences. In eukaryotes, the processing of pre-mRNA and RNA editing take place at sites determined by the base pairing between the target RNA and RNA constituents of small nuclear ribonucleoproteins (snRNPs). Such enzyme targeting is also responsible for gene down regulation through RNA interference (RNAi), where an enzyme-associated guide RNA targets specific mRNA for selective destruction. Likewise, in eukaryotes the maintenance of telomeres involves copying of an RNA template that is a constituent part of the telomerase ribonucleoprotein enzyme. Another cellular organelle, the vault, includes a ribonucleoprotein component, although the function of this organelle remains to be elucidated.
See also
[edit]- GADV-protein world hypothesis
- The Major Transitions in Evolution
- RNA-based evolution
- Protocell or Pre-cell, the primordial version of a cell which confined RNA and later, DNA
- First universal common ancestor (FUCA)
References
[edit]- ^ Johnson, Mark (9 March 2024). "'Monumental' experiment suggests how life on Earth may have started". The Washington Post. Archived from the original on 9 March 2024. Retrieved 10 March 2024.
- ^ a b Neveu M, Kim HJ, Benner SA (April 2013). "The "strong" RNA world hypothesis: fifty years old". Astrobiology. 13 (4): 391–403. Bibcode:2013AsBio..13..391N. doi:10.1089/ast.2012.0868. PMID 23551238.
[The RNA world's existence] has broad support within the community today.
- ^ a b c d e Cech TR (July 2012). "The RNA worlds in context". Cold Spring Harbor Perspectives in Biology. 4 (7): a006742. doi:10.1101/cshperspect.a006742. PMC 3385955. PMID 21441585.
- ^ Rana AK, Ankri S (2016). "Reviving the RNA World: An Insight into the Appearance of RNA Methyltransferases". Frontiers in Genetics. 7: 99. doi:10.3389/fgene.2016.00099. PMC 4893491. PMID 27375676.
- ^ Becker S, Feldmann J, Wiedemann S, Okamura H, Schneider C, Iwan K, et al. (October 2019). "Unified prebiotically plausible synthesis of pyrimidine and purine RNA ribonucleotides". Science. 366 (6461): 76–82. Bibcode:2019Sci...366...76B. doi:10.1126/science.aax2747. PMID 31604305. S2CID 203719976.
- ^ Zimmer, Carl (September 25, 2014). "A Tiny Emissary from the Ancient Past". The New York Times. Archived from the original on September 27, 2014. Retrieved September 26, 2014.
- ^ a b White HB 3rd (1976). "Coenzymes as fossils of an earlier metabolic state". J Mol Evol. 7 (2): 101–104. Bibcode:1976JMolE...7..101W. doi:10.1007/BF01732468. PMID 1263263. S2CID 22282629.
- ^ Patel BH, Percivalle C, Ritson DJ, Duffy CD, Sutherland JD (April 2015). "Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism". Nature Chemistry. 7 (4): 301–307. Bibcode:2015NatCh...7..301P. doi:10.1038/nchem.2202. PMC 4568310. PMID 25803468.
- ^ a b Robertson MP, Joyce GF (May 2012). "The origins of the RNA world". Cold Spring Harbor Perspectives in Biology. 4 (5): a003608. doi:10.1101/cshperspect.a003608. PMC 3331698. PMID 20739415.
- ^ Wade, Nicholas (May 4, 2015). "Making Sense of the Chemistry That Led to Life on Earth". The New York Times. Archived from the original on July 9, 2017. Retrieved May 10, 2015.
- ^ Copley SD, Smith E, Morowitz HJ (December 2007). "The origin of the RNA world: co-evolution of genes and metabolism". Bioorganic Chemistry. 35 (6): 430–443. doi:10.1016/j.bioorg.2007.08.001. PMID 17897696.
The proposal that life on Earth arose from an RNA World is the one most researched in the topic of Abiogenesis.
- ^ Pressman A, Blanco C, Chen IA (October 2015). "The RNA World as a Model System to Study the Origin of Life". Current Biology. 25 (19): R953–R963. Bibcode:2015CBio...25.R953P. doi:10.1016/j.cub.2015.06.016. PMID 26439358. S2CID 43793294.
- ^ Garwood RJ (2012). "Patterns In Palaeontology: The first 3 billion years of evolution". Palaeontology Online. 2 (11): 1–14. Archived from the original on June 26, 2015. Retrieved June 25, 2015.
- ^ Orgel LE (2004). "Prebiotic chemistry and the origin of the RNA world". Critical Reviews in Biochemistry and Molecular Biology. 39 (2): 99–123. doi:10.1080/10409230490460765. PMID 15217990. S2CID 4939632.
- ^ Lehman N (October 2015). "The RNA World: 4,000,000,050 years old". Life. 5 (4): 1583–1586. Bibcode:2015Life....5.1583L. doi:10.3390/life5041583. PMC 4695837. PMID 26791312.
- ^ Rich, Alexander (1962). "On the problems of evolution and biochemical information transfer". In Kasha, Michael; Pullman, Bernard (eds.). Horizons in Biochemistry: Albert Szent-Györgyi Dedicatory Volume. Academic Press. pp. 103–126. ISBN 978-0-12-400450-4.
- ^ Crick FH (December 1968). "The origin of the genetic code". Journal of Molecular Biology. 38 (3): 367–379. doi:10.1016/0022-2836(68)90392-6. PMID 4887876. S2CID 4144681.
- ^ Orgel LE (December 1968). "Evolution of the genetic apparatus". Journal of Molecular Biology. 38 (3): 381–393. doi:10.1016/0022-2836(68)90393-8. PMID 5718557.
- ^ Woese C.R. (1967). The genetic code: The molecular basis for genetic expression. p. 186. Harper & Row
- ^ White HB (March 1976). "Coenzymes as fossils of an earlier metabolic state". Journal of Molecular Evolution. 7 (2): 101–104. Bibcode:1976JMolE...7..101W. doi:10.1007/BF01732468. PMID 1263263. S2CID 22282629.
- ^ a b c d Atkins JF, Gesteland RF, Cech T (2006). The RNA world: the nature of modern RNA suggests a prebiotic RNA world. Plainview, N.Y: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-739-6.
- ^ a b c d Powner MW, Gerland B, Sutherland JD (May 2009). "Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions". Nature. 459 (7244): 239–242. Bibcode:2009Natur.459..239P. doi:10.1038/nature08013. PMID 19444213. S2CID 4412117.
- ^ Orgel L (November 2000). "Origin of life. A simpler nucleic acid". Science. 290 (5495): 1306–1307. doi:10.1126/science.290.5495.1306. PMID 11185405. S2CID 83662769.
- ^ Nelson KE, Levy M, Miller SL (April 2000). "Peptide nucleic acids rather than RNA may have been the first genetic molecule". Proceedings of the National Academy of Sciences of the United States of America. 97 (8): 3868–3871. Bibcode:2000PNAS...97.3868N. doi:10.1073/pnas.97.8.3868. PMC 18108. PMID 10760258.
- ^ Anastasi C, Buchet FF, Crowe MA, Parkes AL, Powner MW, Smith JM, Sutherland JD (April 2007). "RNA: prebiotic product, or biotic invention?". Chemistry & Biodiversity. 4 (4): 721–739. doi:10.1002/cbdv.200790060. PMID 17443885. S2CID 23526930.
- ^ Kruger, Kelly; Grabowski, Paula J.; Zaug, Arthur J.; Sands, Julie; Gottschling, Daniel E.; Cech, Thomas R. (November 1982). "Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of tetrahymena". Cell. 31 (1): 147–157. doi:10.1016/0092-8674(82)90414-7. ISSN 0092-8674. PMID 6297745. S2CID 14787080.
- ^ Guerrier-Takada, Cecilia; Gardiner, Katheleen; Marsh, Terry; Pace, Norman; Altman, Sidney (December 1983). "The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme". Cell. 35 (3): 849–857. doi:10.1016/0092-8674(83)90117-4. ISSN 0092-8674. PMID 6197186. S2CID 39111511.
- ^ Forster AC, Symons RH (April 1987). "Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites". Cell. 49 (2): 211–220. doi:10.1016/0092-8674(87)90562-9. PMID 2436805. S2CID 33415709.
- ^ Johnston WK, Unrau PJ, Lawrence MS, Glasner ME, Bartel DP (May 2001). "RNA-catalyzed RNA polymerization: accurate and general RNA-templated primer extension" (PDF). Science. 292 (5520): 1319–1325. Bibcode:2001Sci...292.1319J. CiteSeerX 10.1.1.70.5439. doi:10.1126/science.1060786. PMID 11358999. S2CID 14174984. Archived (PDF) from the original on 2012-02-27.
- ^ Johnston WK, Unrau PJ, Lawrence MS, Glasner ME, Bartel DP (May 2001). "RNA-catalyzed RNA polymerization: accurate and general RNA-templated primer extension". Science. 292 (5520): 1319–1325. Bibcode:2001Sci...292.1319J. CiteSeerX 10.1.1.70.5439. doi:10.1126/science.1060786. PMID 11358999. S2CID 14174984.
- ^ Zaher HS, Unrau PJ (July 2007). "Selection of an improved RNA polymerase ribozyme with superior extension and fidelity". RNA. 13 (7). New York, N.Y.: 1017–26. doi:10.1261/rna.548807. PMC 1894930. PMID 17586759.
- ^ a b Horning DP, Joyce GF (August 2016). "Amplification of RNA by an RNA polymerase ribozyme". Proceedings of the National Academy of Sciences of the United States of America. 113 (35): 9786–9791. Bibcode:2016PNAS..113.9786H. doi:10.1073/pnas.1610103113. PMC 5024611. PMID 27528667.
- ^ Huang F, Yang Z, Yarus M (November 1998). "RNA enzymes with two small-molecule substrates". Chemistry & Biology. 5 (11): 669–678. doi:10.1016/s1074-5521(98)90294-0. PMID 9831528.
- ^ Unrau PJ, Bartel DP (September 1998). "RNA-catalysed nucleotide synthesis". Nature. 395 (6699): 260–263. Bibcode:1998Natur.395..260U. doi:10.1038/26193. PMID 9751052. S2CID 9734076.
- ^ Gysbers R, Tram K, Gu J, Li Y (June 2015). "Evolution of an Enzyme from a Noncatalytic Nucleic Acid Sequence". Scientific Reports. 5: 11405. Bibcode:2015NatSR...511405G. doi:10.1038/srep11405. PMC 4473686. PMID 26091540.
- ^ Nunes Palmeira R, Colnaghi M, Harrison SA, Pomiankowski A, Lane N (November 2022). "The limits of metabolic heredity in protocells". Proceedings. Biological Sciences. 289 (1986): 20221469. doi:10.1098/rspb.2022.1469. PMC 9653231. PMID 36350219.
- ^ Erives A (August 2011). "A model of proto-anti-codon RNA enzymes requiring L-amino acid homochirality". Journal of Molecular Evolution. 73 (1–2): 10–22. Bibcode:2011JMolE..73...10E. doi:10.1007/s00239-011-9453-4. PMC 3223571. PMID 21779963.
- ^ Noller HF, Hoffarth V, Zimniak L (June 1992). "Unusual resistance of peptidyl transferase to protein extraction procedures". Science. 256 (5062): 1416–1419. Bibcode:1992Sci...256.1416N. doi:10.1126/science.1604315. PMID 1604315.
- ^ Krupkin M, Matzov D, Tang H, Metz M, Kalaora R, Belousoff MJ, et al. (October 2011). "A vestige of a prebiotic bonding machine is functioning within the contemporary ribosome". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 366 (1580): 2972–2978. doi:10.1098/rstb.2011.0146. PMC 3158926. PMID 21930590.
- ^ Bose T, Fridkin G, Davidovich C, Krupkin M, Dinger N, Falkovich AH, et al. (February 2022). "Origin of life: protoribosome forms peptide bonds and links RNA and protein dominated worlds". Nucleic Acids Research. 50 (4): 1815–1828. doi:10.1093/nar/gkac052. PMC 8886871. PMID 35137169.
- ^ Zhang B, Cech TR (November 1997). "Peptide bond formation by in vitro selected ribozymes". Nature. 390 (6655): 96–100. Bibcode:1997Natur.390...96Z. doi:10.1038/36375. PMID 9363898. S2CID 4398830.
- ^ Szathmáry E (June 1999). "The origin of the genetic code: amino acids as cofactors in an RNA world". Trends in Genetics. 15 (6): 223–229. doi:10.1016/S0168-9525(99)01730-8. PMID 10354582.
- ^ Decker, Karl (2006-01-17). "The Pyridine Nucleotide Coenzymes. Herausgegeben von J. Everse, B. Anderson und K.-S. You. Academic Press, New York 1982. XXXV, 389 S., geb. $ 46.00". Angewandte Chemie. 95 (12): 1033–1034. doi:10.1002/ange.19830951241. ISSN 0044-8249.
- ^ Ekland, Eric H.; Szostak, Jack W.; Bartel, David P. (1995-07-21). "Structurally Complex and Highly Active RNA Ligases Derived from Random RNA Sequences". Science. 269 (5222): 364–370. Bibcode:1995Sci...269..364E. doi:10.1126/science.7618102. ISSN 0036-8075. PMID 7618102. S2CID 40795082.
- ^ Tsukiji, Shinya; Pattnaik, Swetansu B; Suga, Hiroaki (2003-08-10). "An alcohol dehydrogenase ribozyme". Nature Structural & Molecular Biology. 10 (9): 713–717. doi:10.1038/nsb964. ISSN 1545-9993. PMID 12910259. S2CID 41081956.
- ^ Tsukiji, Shinya; Pattnaik, Swetansu B.; Suga, Hiroaki (2004-04-06). "Reduction of an Aldehyde by a NADH/Zn2+-Dependent Redox Active Ribozyme". Journal of the American Chemical Society. 126 (16): 5044–5045. doi:10.1021/ja0495213. ISSN 0002-7863. PMID 15099068.
- ^ "Uracil". Archived from the original on 2015-09-08. Retrieved 2020-07-24.
- ^ Lindahl T (April 1993). "Instability and decay of the primary structure of DNA". Nature. 362 (6422): 709–715. Bibcode:1993Natur.362..709L. doi:10.1038/362709a0. PMID 8469282. S2CID 4283694.
- ^ Pääbo S (November 1993). "Ancient DNA". Scientific American. 269 (5): 86–92. Bibcode:1993SciAm.269e..86P. doi:10.1038/scientificamerican1193-86. PMID 8235556. S2CID 5288515.
- ^ Winkler, Wade C.; Breaker, Ronald R. (2005-10-01). "Regulation of Bacterial Gene Expression by Riboswitches". Annual Review of Microbiology. 59 (1): 487–517. doi:10.1146/annurev.micro.59.030804.121336. ISSN 0066-4227. PMID 16153177.
- ^ Nudler E, Mironov AS (January 2004). "The riboswitch control of bacterial metabolism". Trends in Biochemical Sciences. 29 (1): 11–17. doi:10.1016/j.tibs.2003.11.004. PMID 14729327.
- ^ Tucker BJ, Breaker RR (June 2005). "Riboswitches as versatile gene control elements". Current Opinion in Structural Biology. 15 (3): 342–348. doi:10.1016/j.sbi.2005.05.003. PMID 15919195.
- ^ Bocobza SE, Aharoni A (October 2008). "Switching the light on plant riboswitches". Trends in Plant Science. 13 (10): 526–533. Bibcode:2008TPS....13..526B. doi:10.1016/j.tplants.2008.07.004. PMID 18778966.
- ^ Narberhaus F, Waldminghaus T, Chowdhury S (January 2006). "RNA thermometers". FEMS Microbiology Reviews. 30 (1): 3–16. doi:10.1111/j.1574-6976.2005.004.x. PMID 16438677.
- ^ Patton, John T. Editor (2008). Segmented Double-stranded RNA Viruses: Structure and Molecular Biology. Caister Academic Press. Editor's affiliation: Laboratory of Infectious Diseases, NIAID, NIH, Bethesda, MD 20892-8026. ISBN 978-1-904455-21-9
- ^ Bell, Graham: The Basics of Selection. Springer, 1997.[page needed]
- ^ Orgel LE (October 1994). "The origin of life on the earth". Scientific American. 271 (4): 76–83. Bibcode:1994SciAm.271d..76O. doi:10.1038/scientificamerican1094-76. PMID 7524147.
- ^ Levy M, Miller SL (July 1998). "The stability of the RNA bases: implications for the origin of life". Proceedings of the National Academy of Sciences of the United States of America. 95 (14): 7933–7938. Bibcode:1998PNAS...95.7933L. doi:10.1073/pnas.95.14.7933. PMC 20907. PMID 9653118.
- ^ Larralde R, Robertson MP, Miller SL (August 1995). "Rates of decomposition of ribose and other sugars: implications for chemical evolution". Proceedings of the National Academy of Sciences of the United States of America. 92 (18): 8158–8160. Bibcode:1995PNAS...92.8158L. doi:10.1073/pnas.92.18.8158. PMC 41115. PMID 7667262.
- ^ Joyce GF, Visser GM, van Boeckel CA, van Boom JH, Orgel LE, van Westrenen J (1984). "Chiral selection in poly(C)-directed synthesis of oligo(G)". Nature. 310 (5978): 602–604. Bibcode:1984Natur.310..602J. doi:10.1038/310602a0. PMID 6462250. S2CID 4367383.
- ^ Carole Anastasi, Michael A. Crowe, Matthew W. Powner, John D. Sutherland "Direct Assembly of Nucleoside Precursors from Two- and Three-Carbon Units Angewandte Chemie International Edition 45(37):6176–79, 2006.
- ^ Powner MW, Sutherland JD (October 2008). "Potentially prebiotic synthesis of pyrimidine beta-D-ribonucleotides by photoanomerization/hydrolysis of alpha-D-cytidine-2'-phosphate". ChemBioChem. 9 (15): 2386–2387. doi:10.1002/cbic.200800391. PMID 18798212. S2CID 5704391.
- ^ Van Noorden R (2009). "RNA world easier to make". Nature. doi:10.1038/news.2009.471. Archived from the original on 2009-05-16.
- ^ Urquhart J (13 May 2009), "Insight into RNA origins", Chemistry World, Royal Society of Chemistry, archived from the original on 4 October 2015
- ^ Callahan MP, Smith KE, Cleaves HJ, Ruzicka J, Stern JC, Glavin DP, et al. (August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". Proceedings of the National Academy of Sciences of the United States of America. 108 (34): 13995–13998. Bibcode:2011PNAS..10813995C. doi:10.1073/pnas.1106493108. PMC 3161613. PMID 21836052.
- ^ Steigerwald J (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. Archived from the original on 23 June 2015. Retrieved 2011-08-10.
- ^ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. Archived from the original on 5 September 2011. Retrieved 2011-08-09.
- ^ Pearce BK, Pudritz RE, Semenov DA, Henning TK (October 2017). "Origin of the RNA world: The fate of nucleobases in warm little ponds". Proceedings of the National Academy of Sciences of the United States of America. 114 (43): 11327–11332. arXiv:1710.00434. Bibcode:2017PNAS..11411327P. doi:10.1073/pnas.1710339114. PMC 5664528. PMID 28973920.
- ^ Than, Ker (August 29, 2012). "Sugar Found In Space". National Geographic. Archived from the original on July 14, 2015. Retrieved August 31, 2012.
- ^ Staff (August 29, 2012). "Sweet! Astronomers spot sugar molecule near star". AP News. Archived from the original on July 14, 2015. Retrieved August 31, 2012.
- ^ Jørgensen JK, Favre C, Bisschop S, Bourke T, Dishoeck E, Schmalzl M (2012). "Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA" (PDF). The Astrophysical Journal Letters. eprint. 757 (1): L4. arXiv:1208.5498. Bibcode:2012ApJ...757L...4J. doi:10.1088/2041-8205/757/1/L4. S2CID 14205612. Archived (PDF) from the original on 2015-09-24.
- ^ Rivilla, Víctor M.; Jiménez-Serra, Izaskun; Martín-Pintado, Jesús; Colzi, Laura; Tercero, Belén; de Vicente, Pablo; Zeng, Shaoshan; Martín, Sergio; García de la Concepción, Juan; Bizzocchi, Luca; Melosso, Mattia (2022). "Molecular Precursors of the RNA-World in Space: New Nitriles in the G+0.693−0.027 Molecular Cloud". Frontiers in Astronomy and Space Sciences. 9: 876870. arXiv:2206.01053. Bibcode:2022FrASS...9.6870R. doi:10.3389/fspas.2022.876870. ISSN 2296-987X.
- ^ "Building blocks for RNA-based life abound at center of our galaxy". EurekAlert!. 2022-07-08. Retrieved 2022-07-11.
- ^ Cleaves, H. James; Miller, Stanley L. (2001-01-01). "The Nicotinamide Biosynthetic Pathway Is a By-Product of the RNA World". Journal of Molecular Evolution. 52 (1): 73–77. Bibcode:2001JMolE..52...73C. doi:10.1007/s002390010135. ISSN 1432-1432. PMID 11139296. S2CID 25458439.
- ^ Huang, Faqing; Bugg, Charles Walter; Yarus, Michael (2000-12-01). "RNA-Catalyzed CoA, NAD, and FAD Synthesis from Phosphopantetheine, NMN, and FMN". Biochemistry. 39 (50): 15548–15555. doi:10.1021/bi002061f. ISSN 0006-2960. PMID 11112541.
- ^ Lincoln TA, Joyce GF (February 2009). "Self-sustained replication of an RNA enzyme". Science. 323 (5918): 1229–1232. Bibcode:2009Sci...323.1229L. doi:10.1126/science.1167856. PMC 2652413. PMID 19131595.
- "First Examples Of RNA That Replicates Itself Indefinitely Developed By Scripps Scientists". Medical News Today. January 12, 2009. Archived from the original on 2009-07-31.
- ^ Turk RM, Chumachenko NV, Yarus M (March 2010). "Multiple translational products from a five-nucleotide ribozyme". Proceedings of the National Academy of Sciences of the United States of America. 107 (10): 4585–4589. Bibcode:2010PNAS..107.4585T. doi:10.1073/pnas.0912895107. PMC 2826339. PMID 20176971.
- "Scientists create tiny RNA molecule with big implications for life's origins". ScienceDaily (Press release). February 24, 2010.
- ^ Marlaire R (3 March 2015). "NASA Ames Reproduces the Building Blocks of Life in Laboratory". NASA. Archived from the original on 5 March 2015. Retrieved 5 March 2015.
- ^ "New Study Identifies Possible Ancestors of RNA". 2018-09-14. Archived from the original on 2020-11-09.
{{cite web}}: CS1 maint: unfit URL (link) - ^ Fialho DM, Clarke KC, Moore MK, Schuster GB, Krishnamurthy R, Hud NV (February 2018). "Glycosylation of a model proto-RNA nucleobase with non-ribose sugars: implications for the prebiotic synthesis of nucleosides". Organic & Biomolecular Chemistry. 16 (8): 1263–1271. Bibcode:2018OrgBC..16.1263F. doi:10.1039/c7ob03017g. PMID 29308815.
- ^ Cafferty BJ, Fialho DM, Khanam J, Krishnamurthy R, Hud NV (April 2016). "Spontaneous formation and base pairing of plausible prebiotic nucleotides in water". Nature Communications. 7 (1): 11328. Bibcode:2016NatCo...711328C. doi:10.1038/ncomms11328. PMC 4848480. PMID 27108699.
- ^ Holmes, Bob (2012) "First Glimpse at the birth of DNA" (New Scientist April 12, 2012)
- ^ Diemer GS, Stedman KM (June 2012). "A novel virus genome discovered in an extreme environment suggests recombination between unrelated groups of RNA and DNA viruses". Biology Direct. 7 (1): 13. doi:10.1186/1745-6150-7-13. PMC 3372434. PMID 22515485.
- ^ Diener TO (August 1971). "Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA". Virology. 45 (2): 411–428. doi:10.1016/0042-6822(71)90342-4. PMID 5095900.
- ^ "ARS Research Timeline – Tracking the Elusive Viroid". 2006-03-02. Archived from the original on 2007-07-06. Retrieved 2007-07-18.
- ^ Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK (November 1976). "Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures". Proceedings of the National Academy of Sciences of the United States of America. 73 (11): 3852–3856. Bibcode:1976PNAS...73.3852S. doi:10.1073/pnas.73.11.3852. PMC 431239. PMID 1069269.
- ^ Diener TO (December 1989). "Circular RNAs: relics of precellular evolution?". Proceedings of the National Academy of Sciences of the United States of America. 86 (23): 9370–9374. Bibcode:1989PNAS...86.9370D. doi:10.1073/pnas.86.23.9370. PMC 298497. PMID 2480600.
- ^ Daròs JA, Elena SF, Flores R (June 2006). "Viroids: an Ariadne's thread into the RNA labyrinth". EMBO Reports. 7 (6): 593–598. doi:10.1038/sj.embor.7400706. PMC 1479586. PMID 16741503.
- ^ a b Flores R, Gago-Zachert S, Serra P, Sanjuán R, Elena SF (2014). "Viroids: survivors from the RNA world?". Annual Review of Microbiology. 68: 395–414. doi:10.1146/annurev-micro-091313-103416. hdl:10261/107724. PMID 25002087.
- ^ Zimmer, Carl (September 25, 2014). "A Tiny Emissary From the Ancient Past". The New York Times. Archived from the original on November 29, 2014. Retrieved November 22, 2014.
- ^ Diener TO (2016), "Viroids: "living fossils" of primordial RNAs?", Biology Direct, 11 (1): 15, doi:10.1186/s13062-016-0116-7, PMC 4807594, PMID 27016066
- ^ Eigen M, Gardiner W, Schuster P, Winkler-Oswatitsch R (April 1981). "The origin of genetic information". Scientific American. 244 (4): 88–92, 96, et passim. Bibcode:1981SciAm.244a..88H. doi:10.1038/scientificamerican0481-88. PMID 6164094.
- ^ Woese CR (1983). The primary lines of descent and the universal ancestor. Chapter in Bendall DS (1983). Evolution from molecules to men. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-28933-7. pp. 209-233.
- ^ a b Bernstein H, Byerly HC, Hopf FA, Michod RE (October 1984). "Origin of sex". Journal of Theoretical Biology. 110 (3): 323–351. Bibcode:1984JThBi.110..323B. doi:10.1016/S0022-5193(84)80178-2. PMID 6209512.
- ^ Bernstein C, Bernstein H (1991). Aging, sex, and DNA repair. Boston: Academic Press. ISBN 978-0-12-092860-6. see pgs. 293-297
- ^ Lamb RA, Choppin PW (1983). "The gene structure and replication of influenza virus". Annual Review of Biochemistry. 52: 467–506. doi:10.1146/annurev.bi.52.070183.002343. PMID 6351727.
- ^ Barry RD (August 1961). "The multiplication of influenza virus. II. Multiplicity reactivation of ultraviolet irradiated virus". Virology. 14 (4): 398–405. doi:10.1016/0042-6822(61)90330-0. hdl:1885/109240. PMID 13687359.
- ^ Gilker JC, Pavilanis V, Ghys R (June 1967). "Multiplicity reactivation in gamma irradiated influenza viruses". Nature. 214 (5094): 1235–1237. Bibcode:1967Natur.214.1235G. doi:10.1038/2141235a0. PMID 6066111. S2CID 4200194.
- ^ a b c Forterre P (March 2006). "Three RNA cells for ribosomal lineages and three DNA viruses to replicate their genomes: a hypothesis for the origin of cellular domain". Proceedings of the National Academy of Sciences of the United States of America. 103 (10): 3669–3674. Bibcode:2006PNAS..103.3669F. doi:10.1073/pnas.0510333103. PMC 1450140. PMID 16505372.
- ^ Zimmer C (May 2006). "Did DNA come from viruses?". Science. 312 (5775): 870–872. doi:10.1126/science.312.5775.870. PMID 16690855. S2CID 39984425.
- ^ Muller AW (October 2005). "Thermosynthesis as energy source for the RNA World: a model for the bioenergetics of the origin of life". Bio Systems. 82 (1): 93–102. arXiv:q-bio/0501013. Bibcode:2005BiSys..82...93M. doi:10.1016/j.biosystems.2005.06.003. PMID 16024164.
- ^ Kumar A, Sharma S, Maurya RA (2010). "Single Nucleotide-Catalyzed Biomimetic Reductive Amination". Advanced Synthesis and Catalysis. 352 (13): 2227–2232. doi:10.1002/adsc.201000178.
- ^ Zimmer, Carl (September 12, 2013). "A Far-Flung Possibility for the Origin of Life". The New York Times. Archived from the original on July 8, 2015. Retrieved September 12, 2013.
- ^ Webb R (August 29, 2013). "Primordial broth of life was a dry Martian cup-a-soup". New Scientist. Archived from the original on April 24, 2015. Retrieved September 13, 2013.
- ^ Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, et al. (October 1993). "PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules". Nature. 365 (6446): 566–568. Bibcode:1993Natur.365..566E. doi:10.1038/365566a0. PMID 7692304. S2CID 4318153.
- ^ Platts SN. "The PAH World – Discotic polynuclear aromatic compounds as a mesophase scaffolding at the origin of life". Archived from the original on 2011-02-03.
- ^ Allamandola L. "Cosmic Distribution of Chemical Complexity". Archived from the original on 2014-02-27.
- ^ Atkinson, Nancy (2010-10-27). "Buckyballs Could Be Plentiful in the Universe". Universe Today. Archived from the original on 2010-10-29. Retrieved 2010-10-28.
- ^ Cami J, Bernard-Salas J, Peeters E, Malek SE (September 2010). "Detection of C60 and C70 in a young planetary nebula". Science. 329 (5996): 1180–1182. Bibcode:2010Sci...329.1180C. doi:10.1126/science.1192035. PMID 20651118. S2CID 33588270.
- ^ Bernstein MP, Sandford SA, Allamandola LJ, Gillette JS, Clemett SJ, Zare RN (February 1999). "UV irradiation of polycyclic aromatic hydrocarbons in ices: production of alcohols, quinones, and ethers". Science. 283 (5405): 1135–1138. Bibcode:1999Sci...283.1135B. doi:10.1126/science.283.5405.1135. PMID 10024233.
- ^ Steigerwald, Bill; Jones, Nancy; Furukawa, Yoshihiro (18 November 2019). "First Detection of Sugars in Meteorites Gives Clues to Origin of Life". NASA. Retrieved 18 November 2019.
- ^ Furukawa Y, Chikaraishi Y, Ohkouchi N, Ogawa NO, Glavin DP, Dworkin JP, et al. (December 2019). "Extraterrestrial ribose and other sugars in primitive meteorites". Proceedings of the National Academy of Sciences of the United States of America. 116 (49): 24440–24445. Bibcode:2019PNAS..11624440F. doi:10.1073/pnas.1907169116. PMC 6900709. PMID 31740594.
- ^ Kunin V (October 2000). "A system of two polymerases--a model for the origin of life". Origins of Life and Evolution of the Biosphere. 30 (5): 459–466. Bibcode:2000OLEB...30..459K. doi:10.1023/A:1006672126867. PMID 11002892. S2CID 5616924.
- ^ Pascal, Robert (2007), "A scenario starting from the first chemical building blocks", in Reisse, Jacques (ed.), From Suns to Life: A Chronological Approach to the History of Life on Earth, Springer Science & Business Media, pp. 163–166, ISBN 978-0-387-45083-4
- ^ a b "Challenging Assumptions About the Origin of Life". Astrobiology Magazine. 18 September 2013. Archived from the original on 8 May 2014. Retrieved 2014-05-07.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ a b Patel BH, Percivalle C, Ritson DJ, Duffy CD, Sutherland JD (April 2015). "Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism". Nature Chemistry. 7 (4): 301–307. Bibcode:2015NatCh...7..301P. doi:10.1038/nchem.2202. PMC 4568310. PMID 25803468.
- ^ Service, Robert F. (16 March 2015). "Researchers may have solved origin-of-life conundrum". Science (News). Washington, D.C.: American Association for the Advancement of Science. ISSN 1095-9203. Archived from the original on 12 August 2015. Retrieved 2015-07-26.
- ^ Gibard C, Bhowmik S, Karki M, Kim EK, Krishnamurthy R (February 2018). "Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions". Nature Chemistry. 10 (2): 212–217. doi:10.1038/nchem.2878. PMC 6295206. PMID 29359747.
- ^ "Scientists Find Potential "Missing Link" in Chemistry That Led to Life on Earth". Scripps Research Institute. November 6, 2017. Archived from the original on 7 November 2017. Retrieved 7 November 2017.
- ^ Fox GE (September 2010). "Origin and evolution of the ribosome". Cold Spring Harbor Perspectives in Biology. 2 (9): a003483. doi:10.1101/cshperspect.a003483. PMC 2926754. PMID 20534711.
- ^ Fox GE (2016). "Origins and early evolution of the ribosome". In Hernández G, Jagus R (eds.). Evolution of the Protein Synthesis Machinery and Its Regulation. Switzerland: Springer, Cham. pp. 31–60. doi:10.1007/978-3-319-39468-8. ISBN 978-3-319-39468-8. S2CID 27493054.
Further reading
[edit]- Attwater J, Raguram A, Morgunov AS, Gianni E, Holliger P (May 2018). "Ribozyme-catalysed RNA synthesis using triplet building blocks". eLife. 7: e35255. doi:10.7554/eLife.35255. PMC 6003772. PMID 29759114.
- Cairns-Smith AG (1993). Genetic Takeover: And the Mineral Origins of Life. Cambridge University Press. ISBN 978-0-521-23312-5.
- Orgel LE (October 1994). "The origin of life on the earth". Scientific American. 271 (4): 76–83. Bibcode:1994SciAm.271d..76O. doi:10.1038/scientificamerican1094-76. PMID 7524147.
- Orgel LE (2004). "Prebiotic chemistry and the origin of the RNA world". Critical Reviews in Biochemistry and Molecular Biology. 39 (2): 99–123. CiteSeerX 10.1.1.537.7679. doi:10.1080/10409230490460765. PMID 15217990. S2CID 4939632.
- Woolfson A (September 2000). Life Without Genes. London: Flamingo. ISBN 978-0-00-654874-4.
- Vlassov AV, Kazakov SA, Johnston BH, Landweber LF (August 2005). "The RNA world on ice: a new scenario for the emergence of RNA information". Journal of Molecular Evolution. 61 (2): 264–273. Bibcode:2005JMolE..61..264V. doi:10.1007/s00239-004-0362-7. PMID 16044244. S2CID 21096886.
- Engelhart AE, Hud NV (December 2010). "Primitive genetic polymers". Cold Spring Harbor Perspectives in Biology. 2 (12): a002196. doi:10.1101/cshperspect.a002196. PMC 2982173. PMID 20462999.
- Bernhardt HS (July 2012). "The RNA world hypothesis: the worst theory of the early evolution of life (except for all the others)(a)". Biology Direct. 7 (1): 23. doi:10.1186/1745-6150-7-23. PMC 3495036. PMID 22793875.
- Sutherland JD (April 2010). "Ribonucleotides". Cold Spring Harbor Perspectives in Biology. 2 (4): a005439. doi:10.1101/cshperspect.a005439. PMC 2845210. PMID 20452951.
- Camprubí E, de Leeuw JW, House CH, Raulin F, Russell MJ, Spang A, Tirumalai MR, Westall F (Dec 2019). "The Emergence of Life". Space Sci Rev. 215 (56): 56. Bibcode:2019SSRv..215...56C. doi:10.1007/s11214-019-0624-8.
- Forster, Anthony C. (2022-05-03). "Revisiting the Extinction of the RNA World". Biochemistry. 61 (9): 749–751. doi:10.1021/acs.biochem.2c00121. PMC 9069686. PMID 35389627.
External links
[edit]- "Understanding the RNA World". Exploring Life's Origins. Exploring Origins Project.
- Ferris, James P. "The Formation of the RNA World". The New York Center for Studies of the Origins of Life, Rensselaer Polytechnic Institute. Archived from the original on March 1, 2012.
- Altman, Sidney (2001). "The RNA World". NobelPrize.org. Nobel Media.
- Kuska, Robert (June 2002). "A World Apart" (PDF). HHMI Bulletin. Howard Hughes Medical Institute. pp. 14–19. Archived (PDF) from the original on 2004-05-22.
- Cech, Thomas R. (2004). "Exploring the New RNA World". NobelPrize.org. Nobel Media.
- Sutherland JD (April 2010). "Ribonucleotides". Cold Spring Harbor Perspectives in Biology. 2 (4): a005439. doi:10.1101/cshperspect.a005439. PMC 2845210. PMID 20452951.
- "The Origins of the RNA World". YouTube. Library of Congress. August 5, 2016.

