Protected percutaneous coronary intervention
| Protected percutaneous coronary intervention | |
|---|---|
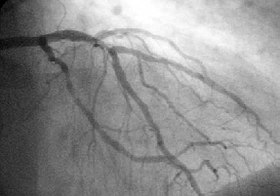 A coronary angiogram delineating the left coronary circulation. |
Protected percutaneous coronary intervention, abbreviated as Protected PCI, is a heart procedure that involves a ventricular assist device that is used to treat patients with cardiovascular disease, including advanced heart failure.[1][2]
Procedure
[edit]During a protected percutaneous coronary intervention (Protected PCI) procedure, "the Impella 2.5 heart pump helps maintain a stable heart function by pumping blood for the heart. This gives a weak heart muscle an opportunity to rest and reduces the heart’s workload, preventing the heart from being overstressed by the procedure as coronary artery blockages are repaired".[3] Deborah Moore, the Director of Interventional Cardiology at Florida Hospital Zephyrhills, stated that protected PCI is beneficial in patients who are "inoperable or non-candidates for stents and angioplasty."[3] The Impella 2.5, used in protected PCI, "received FDA approval for elective and urgent high-risk PCI procedures in March 2015, following its 510(k) clearance in 2008" and as a percutaneous hemodynamic support device, it was deemed "safe and effective for patients with complex coronary disease, depressed ejection fraction, other co-morbidities, and who have been refused for surgical treatment."[4] The PROTECT II Study, published in Circulation found that the "Impella 2.5 provided superior hemodynamic support in comparison with IABP, with maximal decrease in cardiac power output from baseline of −0.04±0.24 W in comparison with −0.14±0.27 W for IABP (P=0.001)."[5] Hospitals such as Detroit Medical Center, and organizations such as the Society of Cardiovascular Angiography and Interventions, have featured lectures including "The New Era of Protected PCI: Treating Elective and High-Risk PCI Patients with Impella 2.5",[6] and "Expanding your Patient Practice with Protected PCI: Treating the Most Complex Patients" in order to educate physicians on using protected percutaneous coronary intervention, respectively.[7]
See also
[edit]References
[edit]- ^ Remondino, Andrea; Christian Seiler; Pascal Meier; Roby D. Rakhit; Kerstin Wustmann; Rainer Zbinden; Stephan Zbinden; Stephan Windecker; Mario Togni; Peter Wenaweser; Bernhard Meier; Franz R. Eberli (2011). "Collateral function changes during primary percutaneous coronary intervention in acute myocardial infarction". Cardiovascular Medicine. 14 (1): 119–126. doi:10.4414/cvm.2011.01579.
This is so because the group undergoing protected primary PCI presented unaltered CFI during the second as compared to the first coronary occlusion.
- ^ Hozawa, Koji; Hitoshi Nakamura; Kazutoshi Yamamoto; Mizuki Hirose; Takanori Miyauchi; Akihiko Kanazawa; Sunao Nakamura; Osamu Hirashima; Nobuyuki Makishima; Shotaro Nakamura; Jun Koyama; Noriki Kutsukata; Makoto Anzai; Akira Honma (2004). "Successful Embolization Protection Using GuardWire System tor Acute Myocardial lntarction". Circulation Journal. 68 (1).
Distal embolism is one of the major complications during percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI). The Guard Wire system (GS) consists of an occlusion balloon which is inflated distally allowing protected PCI has recently become available.
- ^ a b "Florida Hospital Zephyrhills Delivers New Game-Changing Technology for Advanced Heart Failure Patients with Coronary Artery Disease". Fox News. 30 September 2015.
{{cite web}}: Missing or empty|url=(help) - ^ Pearson, Dave; Mary Tierney (22 September 2015). "Building a Protected PCI Program: The Heart Team Approach". Cardiovascular Business. Retrieved 13 October 2015.
Einstein's Protected PCI program has operated from a position of strength, but expansion this spring broadened the patient mix to include elective and urgent high-risk PCI patients. At the center of the program is the Impella 2.5. The device received FDA approval for elective and urgent high-risk PCI procedures in March 2015, following its 510(k) clearance in 2008, making it the first percutaneous hemodynamic support device to get the regulatory nod as both safe and effective for patients with complex coronary disease, depressed ejection fraction, other co-morbidities, and who have been refused for surgical treatment.
- ^ O'Neill, W. W.; N. S. Kleiman; J. Moses; J. P. S. Henriques; S. Dixon; J. Massaro; I. Palacios; B. Maini; S. Mulukutla; V. Dzavik; J. Popma; P. S. Douglas; M. Ohman (2012). "A Prospective, Randomized Clinical Trial of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention: The PROTECT II Study". Circulation. 126 (14): 1717–1727. doi:10.1161/CIRCULATIONAHA.112.098194. ISSN 0009-7322. PMID 22935569.
- ^ "The New Era of Protected PCI: Treating Elective and High-Risk PCI Patients with Impella 2.5". Cath Lab Digest. 11 September 2015.
{{cite web}}: Missing or empty|url=(help) - ^ "Expanding your Patient Practice with Protected PCI: Treating the Most Complex Patients". The Society for Cardiovascular Angiography and Interventions. 6 May 2015.
{{cite web}}: Missing or empty|url=(help)
