C2-Symmetric ligands
In homogeneous catalysis, C2-symmetric ligands refer to ligands that lack mirror symmetry but have C2 symmetry (two-fold rotational symmetry). Such ligands are usually bidentate and are valuable in catalysis.[1] The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. C2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including C2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.
Examples
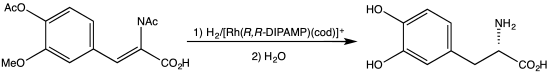
[edit]An early C2-symmetric ligand, diphosphine catalytic ligand DIPAMP, was developed in 1968 by William S. Knowles and coworkers of Monsanto Company, who shared the 2001 Nobel Prize in Chemistry.[2] This ligand was used in the industrial production of L-DOPA.
Some classes of C2-symmetric ligands are called privileged ligands, which are ligands that are broadly applicable to multiple catalytic processes, not only a single reaction type.[3][4]
- Ligands and Complexes
-
DuPhos ligands are a class of C2-symmetric ligands for asymmetric hydrogenation.[6]
-
Oxaliplatin, containing the C2-symmetric (R,R)-diaminocyclohexane ligand, is an important anticancer drug.
-
Jacobsen's epoxidation catalyst is a complex of a C2-symmetric salen-type ligand.
-
C2-symmetric diene ligand.[7]
-
Both bi- and tridentate bis(oxazoline) ligands are used in organic synthesis
-
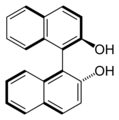
Both enantiomers of BINAP
-
BINOL, another binaphthalene-based ligand
-
DIPAMP, a diphosphine of historic significance
Mechanistic concepts
[edit]While the presence of any symmetry element within a ligand intended for asymmetric induction might appear counterintuitive, asymmetric induction only requires that the ligand be chiral (i.e. have no improper rotation axis). Asymmetry (i.e. absence of any symmetry elements) is not required. C2 symmetry improves the enantioselectivity of the complex by reducing the number of unique geometries in the transition states. Steric and kinetic factors then usually favor the formation of a single product.[1][8]

Chiral fence
[edit]Chiral ligands work by asymmetric induction somewhere along the reaction coordinate. The image to the right illustrates how a chiral ligand may induce an enantioselective reaction. The ligand (in green) has C2 symmetry with its nitrogen, oxygen or phosphorus atoms hugging a central metal atom (in red). In this particular ligand the right side is sticking out and its left side points away. The substrate in this reduction is acetophenone and the reagent (in blue) a hydride ion. In absence of the metal and the ligand the Re face approach of the hydride ion gives the (S)-enantiomer and the Si face approach the (R)-enantiomer in equal amounts (a racemic mixture like expected). The ligand and metal presence changes all that. The carbonyl group will coordinate with the metal and due to the steric bulk of the phenyl group it will only be able to do so with its Si face exposed to the hydride ion with in the ideal situation exclusive formation of the (R) enantiomer. The re face will simply hit the chiral fence.[9] Note that when the ligand is replaced by its mirror image the other enantiomer will form and that a racemic mixture of ligand will once again yield a racemic product. Also note that if the steric bulk of both carbonyl substituents is very similar the strategy will fail.
Other C2-symmetric complexes
[edit]Many C2-symmetric complexes are known. Some arise not from C2-symmetric ligands, but from the orientation or disposition of high symmetry ligands within the coordination sphere of the metal. Notably, EDTA and triethylenetetraamine form complexes that are C2-symmetric by virtue of the way the ligands wrap around the metal centers. Two isomers are possible for (indenyl)2MX2, Cs- and C2-symmetric. The C2-symmetric complexes are optically stable.
Asymmetric ligands
[edit]Ligands containing atomic chirality centers such asymmetric carbon, which usually do not have C2-symmetry, remain important in catalysis. Examples include cinchona alkaloids and certain phosphoramidites. P-chiral monophosphines have also been investigated.
See also
[edit]Further reading
[edit]- Desimoni, G.; Faita, G.; Jorgensen, K. A. (2006). "C2-Symmetric Chiral Bis(Oxazoline) Ligands in Asymmetric Catalysis". Chem. Rev. 106 (9): 3561–3651. doi:10.1021/cr0505324. PMID 16967916.
- Liu, X.; Lin, L.; Feng, X. (2011). "Chiral N,N'-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions". Acc. Chem. Res. 44 (8): 574–587. doi:10.1021/ar200015s. PMID 21702458.
- Evans, D. A.; Kozlowski, M. C.; Murry, J. A.; Burgey, C. S.; Campos, K. R.; Connell, B. T.; Staples, R. J. (1999). "C2-Symmetric Copper(II) Complexes as Chiral Lewis Acids. Scope and Mechanism of Catalytic Enantioselective Aldol Additions of Enolsilanes to (Benzyloxy)Acetaldehyde". J. Am. Chem. Soc. 121 (4): 669–685. doi:10.1021/JA9829822.
- Gao, J.-X.; Ikariya, T.; Noyori, R. (1996). "A Ruthenium(II) Complex with a C2-Symmetric Diphosphine/Diamine Tetradentate Ligand for Asymmetric Transfer Hydrogenation of Aromatic Ketones". Organometallics. 15 (4): 1087–1089. doi:10.1021/OM950833B.
- Pye, P. J.; Rossen, K.; Reamer, R. A.; Tsou, N. N.; Volante, R. P.; Reider, P. J. (1997). "New Planar Chiral Bisphosphine Ligand for Asymmetric Catalysis: Highly Enantioselective Hydrogenations under Mild Conditions". J. Am. Chem. Soc. 119 (26): 6207–6208. doi:10.1021/JA970654G.
References
[edit]- ^ a b James K. Whitesell (1989). "C2 Symmetry and Symmetric Induction". Chem. Rev. 89 (7): 1581–1590. doi:10.1021/cr00097a012.
- ^ Nobel prize 2001 www.nobelprize.org Link Archived 2007-07-13 at the Wayback Machine
- ^ Pfaltz, A. (2004). "Asymmetric Catalysis Special Feature Part II: Design of chiral ligands for asymmetric catalysis: From C2-symmetric P,P- and N,N-ligands to sterically and electronically nonsymmetrical P,N-ligands". Proceedings of the National Academy of Sciences. 101 (16): 5723–5726. Bibcode:2004PNAS..101.5723P. doi:10.1073/pnas.0307152101. PMC 395974. PMID 15069193.
- ^ Yoon, TP; Jacobsen, EN (March 2003). "Privileged chiral catalysts". Science. 299 (5613): 1691–3. Bibcode:2003Sci...299.1691Y. doi:10.1126/science.1083622. PMID 12637734. S2CID 27416160.
- ^ Dang, T. P.; Kagan, H. B. (1971). "The asymmetric synthesis of hydratropic acid and amino-acids by homogeneous catalytic hydrogenation". Journal of the Chemical Society D: Chemical Communications (10): 481. doi:10.1039/C29710000481.
- ^ Burk, M. J.; Feaster, J. E.; Nugent, W. A.; Harlow, R. L. (1993). "Preparation and Use of C2-Symmetric Bis(Phospholanes): Production of a-Amino Acid Derivatives Via Highly Enantioselective Hydrogenation Reactions". J. Am. Chem. Soc. 115: 10125–10138. doi:10.1021/ja00075a031.
- ^ Hayashi, T.; Ueyama, K.; Tokunaga, N.; Yoshida, K. (2003). "A Chiral Chelating Diene as a New Type of Chiral Ligand for Transition Metal Catalysts: Its Preparation and Use for the Rhodium-Catalyzed Asymmetric 1,4-Addition". J. Am. Chem. Soc. 125 (38): 11508–11509. doi:10.1021/ja037367z. PMID 13129348.
- ^ Rasappan, Ramesh; Laventine, Dominic; Reiser, Oliver (2008). "Metal-bis(oxazoline) complexes: From coordination chemistry to asymmetric catalysis". Coordination Chemistry Reviews. 252 (5–7): 702–714. doi:10.1016/j.ccr.2007.11.007.
- ^ Hisao, Nishiyama (1989). "Chiral and C2-symmetrical bis(oxazolinylpyridine)rhodium(III) complexes: effective catalysts for asymmetric hydrosilylation of ketones". Organometallics. 8 (3): 846–848. doi:10.1021/om00105a047.

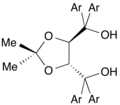
![The C2-symmetric diphosphine DIOP is historically significant.[5]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/95/%28S%2CS%29-DIOP.svg/120px-%28S%2CS%29-DIOP.svg.png)
![DuPhos ligands are a class of C2-symmetric ligands for asymmetric hydrogenation.[6]](http://upload.wikimedia.org/wikipedia/commons/thumb/2/2c/DuPhos_ligands.svg/120px-DuPhos_ligands.svg.png)


![C2-symmetric diene ligand.[7]](http://upload.wikimedia.org/wikipedia/commons/thumb/0/0d/HayashiChiralNBD.svg/120px-HayashiChiralNBD.svg.png)