Peroxyacyl nitrates
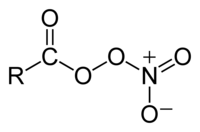

In organic chemistry, peroxyacyl nitrates (also known as Acyl peroxy nitrates, APN or PANs) are powerful respiratory and eye irritants present in photochemical smog. They are nitrates produced in the thermal equilibrium between organic peroxy radicals by the gas-phase oxidation of a variety of volatile organic compounds (VOCs), or by aldehydes and other oxygenated VOCs oxidizing in the presence of NO2.[1][2][3]
For example, peroxyacetyl nitrate, CH3C(O)OONO2:
- Hydrocarbons + O2 + NO2 + light → CH3COOONO2
The general equation is:
- CxHyO3 + NO2 → CxHyO3NO2
They are good markers for the source of VOCs as either biogenic or anthropogenic, which is useful in the study of global and local effects of pollutants.[4][5]
PANs are both toxic and irritating, as they dissolve more readily in water than ozone. They are lachrymators, causing eye irritation at concentrations of only a few parts per billion. At higher concentrations they cause extensive damage to vegetation. Both PANs and their chlorinated derivates are said to be mutagenic, as they can be a factor causing skin cancer.
PANs are secondary pollutants, which means they are not directly emitted as exhaust from power plants or internal combustion engines, but they are formed from other pollutants by chemical reactions in the atmosphere. Free radical reactions catalyzed by ultraviolet light from the sun oxidize unburned hydrocarbons to aldehydes, ketones, and dicarbonyl compounds, whose secondary reactions create peroxyacyl radicals, which combine with nitrogen dioxide to form peroxyacyl nitrates.
The most common peroxyacyl radical is peroxyacetyl, which can be formed from the free radical oxidation of acetaldehyde, various ketones, or the photolysis of dicarbonyl compounds such as methylglyoxal or diacetyl.
Since they dissociate quite slowly in the atmosphere into radicals and NO2, PANs are able to transport these unstable compounds far away from the urban and industrial origin. This is important for tropospheric ozone production as PANs transport NOx to regions where it can more efficiently produce ozone.
References
[edit]- ^ Cape, J.N. (2003). "Effects of airborne volatile organic compounds on plants". Environmental Pollution. 122 (1): 145–157. doi:10.1016/S0269-7491(02)00273-7. PMID 12535603.
- ^ Gaffney, Jeffrey S.; Marley, Nancy A. (2021). "The Impacts of Peroxyacetyl Nitrate in the Atmosphere of Megacities and Large Urban Areas: A Historical Perspective". ACS Earth and Space Chemistry. 5 (8): 1829–1841. Bibcode:2021ESC.....5.1829G. doi:10.1021/acsearthspacechem.1c00143. S2CID 238708473.
- ^ Jickells, T.; Baker, A. R.; Cape, J. N.; Cornell, S. E.; Nemitz, E. (2013). "The cycling of organic nitrogen through the atmosphere". Philosophical Transactions of the Royal Society B: Biological Sciences. 368 (1621). doi:10.1098/rstb.2013.0115. PMC 3682737. PMID 23713115.
- ^ LaFranchi, B. W.; Wolfe, G. M. (2009). "Closing the peroxy acetyl nitrate budget: observations of acyl peroxy nitrates (PAN, PPN, and MPAN) during BEARPEX 200" (PDF). Atmos. Chem. Phys. 9 (19). Copernicus Publications: 7623–7641. Bibcode:2009ACP.....9.7623L. doi:10.5194/acp-9-7623-2009.
- ^ Thornton, Joel. "PANs". Department of Atmospheric Sciences, University of Washington. Retrieved 14 November 2010.
