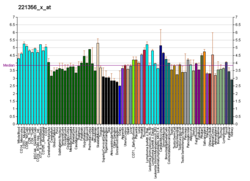
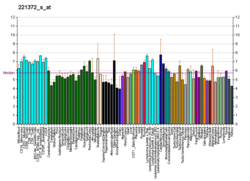
P2RX2
Appearance
(Redirected from P2X2)
P2X purinoceptor 2 is a protein that in humans is encoded by the P2RX2 gene.[5][6][7]
The product of this gene belongs to the family of purinoceptors for ATP. This receptor functions as a cation conducting ligand-gated ion channel. Binding to ATP mediates synaptic transmission between neurons and from neurons to smooth muscle. Six transcript variants encoding six distinct isoforms have been identified for this gene.[7]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000187848 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000029503 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Lynch KJ, Touma E, Niforatos W, Kage KL, Burgard EC, van Biesen T, Kowaluk EA, Jarvis MF (Dec 1999). "Molecular and functional characterization of human P2X(2) receptors". Mol Pharmacol. 56 (6): 1171–81. doi:10.1124/mol.56.6.1171. PMID 10570044.
- ^ Brake AJ, Wagenbach MJ, Julius D (Oct 1994). "New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor". Nature. 371 (6497): 519–23. Bibcode:1994Natur.371..519B. doi:10.1038/371519a0. PMID 7523952. S2CID 4371044.
- ^ a b "Entrez Gene: P2RX2 purinergic receptor P2X, ligand-gated ion channel, 2".
Further reading
[edit]- North RA (2002). "Molecular physiology of P2X receptors". Physiol. Rev. 82 (4): 1013–67. doi:10.1152/physrev.00015.2002. PMID 12270951.
- Barrera NP, Ormond SJ, Henderson RM, et al. (2005). "Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize". J. Biol. Chem. 280 (11): 10759–65. doi:10.1074/jbc.M412265200. PMID 15657042.
- Mason HS, Bourke S, Kemp PJ (2005). "Selective modulation of ligand-gated P2X purinoceptor channels by acute hypoxia is mediated by reactive oxygen species". Mol. Pharmacol. 66 (6): 1525–35. doi:10.1124/mol.104.000851. PMID 15331767. S2CID 24536809.
- Aschrafi A, Sadtler S, Niculescu C, et al. (2004). "Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes". J. Mol. Biol. 342 (1): 333–43. doi:10.1016/j.jmb.2004.06.092. PMID 15313628.
- Boué-Grabot E, Emerit MB, Toulmé E, et al. (2004). "Cross-talk and co-trafficking between rho1/GABA receptors and ATP-gated channels". J. Biol. Chem. 279 (8): 6967–75. doi:10.1074/jbc.M307772200. PMID 14660627.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Järlebark LE, Housley GD, Raybould NP, et al. (2003). "ATP-gated ion channels assembled from P2X2 receptor subunits in the mouse cochlea". NeuroReport. 13 (15): 1979–84. doi:10.1097/00001756-200210280-00030. PMID 12395104. S2CID 10991307.
- Khakh BS, Zhou X, Sydes J, et al. (2000). "State-dependent cross-inhibition between transmitter-gated cation channels" (PDF). Nature. 406 (6794): 405–10. Bibcode:2000Natur.406..405K. doi:10.1038/35019066. PMID 10935636. S2CID 4500837.
- Brändle U, Spielmanns P, Osteroth R, et al. (1997). "Desensitization of the P2X(2) receptor controlled by alternative splicing". FEBS Lett. 404 (2–3): 294–8. Bibcode:1997FEBSL.404..294B. doi:10.1016/S0014-5793(97)00128-2. PMID 9119082. S2CID 39951168.
- Lewis C, Neidhart S, Holy C, et al. (1995). "Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons". Nature. 377 (6548): 432–5. Bibcode:1995Natur.377..432L. doi:10.1038/377432a0. PMID 7566120. S2CID 4361850.
External links
[edit]- P2RX2+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.