Nihonium
| Nihonium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /nɪˈhoʊniəm/ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass number | [286] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nihonium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 113 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 13 (boron group) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d10 7s2 7p1 (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 3 (predicted) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid (predicted)[1][2][3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 700 K (430 °C, 810 °F) (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1430 K (1130 °C, 2070 °F) (predicted)[1][4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 16 g/cm3 (predicted)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 7.61 kJ/mol (extrapolated)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporisation | 130 kJ/mol (predicted)[2][4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: (none) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionisation energies | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 170 pm (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 172–180 pm (extrapolated)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | synthetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) (predicted)[5][6] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 54084-70-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | After Japan (Nihon in Japanese) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Riken (Japan, first undisputed claim 2004) JINR (Russia) and Livermore (US, first announcement 2003) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of nihonium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nihonium is a synthetic chemical element; it has the symbol Nh and atomic number 113. It is extremely radioactive: its most stable known isotope, nihonium-286, has a half-life of about 10 seconds. In the periodic table, nihonium is a transactinide element in the p-block. It is a member of period 7 and group 13.
Nihonium was first reported to have been created in experiments carried out between 14 July and 10 August 2003, by a Russian–American collaboration at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, working in collaboration with the Lawrence Livermore National Laboratory in Livermore, California,[10] and on 23 July 2004, by a team of Japanese scientists at Riken in Wakō, Japan.[11] The confirmation of their claims in the ensuing years involved independent teams of scientists working in the United States, Germany, Sweden, and China, as well as the original claimants in Russia and Japan. In 2015, the IUPAC/IUPAP Joint Working Party recognised the element and assigned the priority of the discovery and naming rights for the element to Riken.[12] The Riken team suggested the name nihonium in 2016, which was approved in the same year. The name comes from the common Japanese name for Japan (日本, nihon).
Very little is known about nihonium, as it has been made only in very small amounts that decay within seconds. The anomalously long lives of some superheavy nuclides, including some nihonium isotopes, are explained by the "island of stability" theory. Experiments to date have supported the theory, with the half-lives of the confirmed nihonium isotopes increasing from milliseconds to seconds as neutrons are added and the island is approached. Nihonium has been calculated to have similar properties to its homologues boron, aluminium, gallium, indium, and thallium. All but boron are post-transition metals, and nihonium is expected to be a post-transition metal as well. It should also show several major differences from them; for example, nihonium should be more stable in the +1 oxidation state than the +3 state, like thallium, but in the +1 state nihonium should behave more like silver and astatine than thallium. Preliminary experiments in 2017 showed that elemental nihonium is not very volatile; its chemistry remains largely unexplored.
Introduction
[edit]Synthesis of superheavy nuclei
[edit]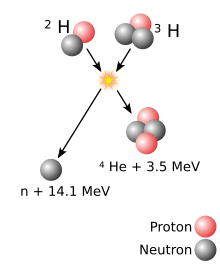
A superheavy[a] atomic nucleus is created in a nuclear reaction that combines two other nuclei of unequal size[b] into one; roughly, the more unequal the two nuclei in terms of mass, the greater the possibility that the two react.[18] The material made of the heavier nuclei is made into a target, which is then bombarded by the beam of lighter nuclei. Two nuclei can only fuse into one if they approach each other closely enough; normally, nuclei (all positively charged) repel each other due to electrostatic repulsion. The strong interaction can overcome this repulsion but only within a very short distance from a nucleus; beam nuclei are thus greatly accelerated in order to make such repulsion insignificant compared to the velocity of the beam nucleus.[19] The energy applied to the beam nuclei to accelerate them can cause them to reach speeds as high as one-tenth of the speed of light. However, if too much energy is applied, the beam nucleus can fall apart.[19]
Coming close enough alone is not enough for two nuclei to fuse: when two nuclei approach each other, they usually remain together for about 10−20 seconds and then part ways (not necessarily in the same composition as before the reaction) rather than form a single nucleus.[19][20] This happens because during the attempted formation of a single nucleus, electrostatic repulsion tears apart the nucleus that is being formed.[19] Each pair of a target and a beam is characterized by its cross section—the probability that fusion will occur if two nuclei approach one another expressed in terms of the transverse area that the incident particle must hit in order for the fusion to occur.[c] This fusion may occur as a result of the quantum effect in which nuclei can tunnel through electrostatic repulsion. If the two nuclei can stay close past that phase, multiple nuclear interactions result in redistribution of energy and an energy equilibrium.[19]
| External videos | |
|---|---|
The resulting merger is an excited state[23]—termed a compound nucleus—and thus it is very unstable.[19] To reach a more stable state, the temporary merger may fission without formation of a more stable nucleus.[24] Alternatively, the compound nucleus may eject a few neutrons, which would carry away the excitation energy; if the latter is not sufficient for a neutron expulsion, the merger would produce a gamma ray. This happens in about 10−16 seconds after the initial nuclear collision and results in creation of a more stable nucleus.[24] The definition by the IUPAC/IUPAP Joint Working Party (JWP) states that a chemical element can only be recognized as discovered if a nucleus of it has not decayed within 10−14 seconds. This value was chosen as an estimate of how long it takes a nucleus to acquire electrons and thus display its chemical properties.[25][d]
Decay and detection
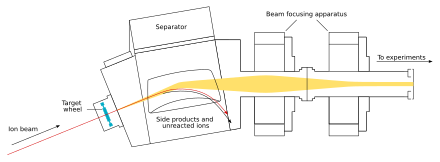
[edit]The beam passes through the target and reaches the next chamber, the separator; if a new nucleus is produced, it is carried with this beam.[27] In the separator, the newly produced nucleus is separated from other nuclides (that of the original beam and any other reaction products)[e] and transferred to a surface-barrier detector, which stops the nucleus. The exact location of the upcoming impact on the detector is marked; also marked are its energy and the time of the arrival.[27] The transfer takes about 10−6 seconds; in order to be detected, the nucleus must survive this long.[30] The nucleus is recorded again once its decay is registered, and the location, the energy, and the time of the decay are measured.[27]
Stability of a nucleus is provided by the strong interaction. However, its range is very short; as nuclei become larger, its influence on the outermost nucleons (protons and neutrons) weakens. At the same time, the nucleus is torn apart by electrostatic repulsion between protons, and its range is not limited.[31] Total binding energy provided by the strong interaction increases linearly with the number of nucleons, whereas electrostatic repulsion increases with the square of the atomic number, i.e. the latter grows faster and becomes increasingly important for heavy and superheavy nuclei.[32][33] Superheavy nuclei are thus theoretically predicted[34] and have so far been observed[35] to predominantly decay via decay modes that are caused by such repulsion: alpha decay and spontaneous fission.[f] Almost all alpha emitters have over 210 nucleons,[37] and the lightest nuclide primarily undergoing spontaneous fission has 238.[38] In both decay modes, nuclei are inhibited from decaying by corresponding energy barriers for each mode, but they can be tunneled through.[32][33]
Alpha particles are commonly produced in radioactive decays because the mass of an alpha particle per nucleon is small enough to leave some energy for the alpha particle to be used as kinetic energy to leave the nucleus.[40] Spontaneous fission is caused by electrostatic repulsion tearing the nucleus apart and produces various nuclei in different instances of identical nuclei fissioning.[33] As the atomic number increases, spontaneous fission rapidly becomes more important: spontaneous fission partial half-lives decrease by 23 orders of magnitude from uranium (element 92) to nobelium (element 102),[41] and by 30 orders of magnitude from thorium (element 90) to fermium (element 100).[42] The earlier liquid drop model thus suggested that spontaneous fission would occur nearly instantly due to disappearance of the fission barrier for nuclei with about 280 nucleons.[33][43] The later nuclear shell model suggested that nuclei with about 300 nucleons would form an island of stability in which nuclei will be more resistant to spontaneous fission and will primarily undergo alpha decay with longer half-lives.[33][43] Subsequent discoveries suggested that the predicted island might be further than originally anticipated; they also showed that nuclei intermediate between the long-lived actinides and the predicted island are deformed, and gain additional stability from shell effects.[44] Experiments on lighter superheavy nuclei,[45] as well as those closer to the expected island,[41] have shown greater than previously anticipated stability against spontaneous fission, showing the importance of shell effects on nuclei.[g]
Alpha decays are registered by the emitted alpha particles, and the decay products are easy to determine before the actual decay; if such a decay or a series of consecutive decays produces a known nucleus, the original product of a reaction can be easily determined.[h] (That all decays within a decay chain were indeed related to each other is established by the location of these decays, which must be in the same place.)[27] The known nucleus can be recognized by the specific characteristics of decay it undergoes such as decay energy (or more specifically, the kinetic energy of the emitted particle).[i] Spontaneous fission, however, produces various nuclei as products, so the original nuclide cannot be determined from its daughters.[j]
The information available to physicists aiming to synthesize a superheavy element is thus the information collected at the detectors: location, energy, and time of arrival of a particle to the detector, and those of its decay. The physicists analyze this data and seek to conclude that it was indeed caused by a new element and could not have been caused by a different nuclide than the one claimed. Often, provided data is insufficient for a conclusion that a new element was definitely created and there is no other explanation for the observed effects; errors in interpreting data have been made.[k]History
[edit]Early indications
[edit]The syntheses of elements 107 to 112 were conducted at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany, from 1981 to 1996. These elements were made by cold fusion[l] reactions, in which targets made of lead and bismuth, which are around the stable configuration of 82 protons, are bombarded with heavy ions of period 4 elements. This creates fused nuclei with low excitation energies due to the stability of the targets' nuclei, significantly increasing the yield of superheavy elements. Cold fusion was pioneered by Yuri Oganessian and his team in 1974 at the Joint Institute for Nuclear Research (JINR) in Dubna, Soviet Union. Yields from cold fusion reactions were found to decrease significantly with increasing atomic number; the resulting nuclei were severely neutron-deficient and short-lived. The GSI team attempted to synthesise element 113 via cold fusion in 1998 and 2003, bombarding bismuth-209 with zinc-70; both attempts were unsuccessful.[59][60]
Faced with this problem, Oganessian and his team at the JINR turned their renewed attention to the older hot fusion technique, in which heavy actinide targets were bombarded with lighter ions. Calcium-48 was suggested as an ideal projectile, because it is very neutron-rich for a light element (combined with the already neutron-rich actinides) and would minimise the neutron deficiencies of the nuclides produced. Being doubly magic, it would confer benefits in stability to the fused nuclei. In collaboration with the team at the Lawrence Livermore National Laboratory (LLNL) in Livermore, California, United States, they made an attempt on element 114 (which was predicted to be a magic number, closing a proton shell, and more stable than element 113).[59]
In 1998, the JINR–LLNL collaboration started their attempt on element 114, bombarding a target of plutonium-244 with ions of calcium-48:[59]
- 244
94Pu
+ 48
20Ca
→ 292114* → 290114 + 2
n
+ e− → 290113 + νe ?
A single atom was observed which was thought to be the isotope 289114: the results were published in January 1999.[61] Despite numerous attempts to repeat this reaction, an isotope with these decay properties has never again been found, and the exact identity of this activity is unknown.[62] A 2016 paper by Sigurd Hofmann et al. considered that the most likely explanation of the 1998 result is that two neutrons were emitted by the produced compound nucleus, leading to 290114 and electron capture to 290113, while more neutrons were emitted in all other produced chains. This would have been the first report of a decay chain from an isotope of element 113, but it was not recognised at the time, and the assignment is still uncertain.[9] A similar long-lived activity observed by the JINR team in March 1999 in the 242Pu + 48Ca reaction may be due to the electron-capture daughter of 287114, 287113; this assignment is also tentative.[8]
JINR–LLNL collaboration
[edit]The now-confirmed discovery of element 114 was made in June 1999 when the JINR team repeated the first 244Pu + 48Ca reaction from 1998;[63][64] following this, the JINR team used the same hot fusion technique to synthesize elements 116 and 118 in 2000 and 2002 respectively via the 248Cm + 48Ca and 249Cf + 48Ca reactions. They then turned their attention to the missing odd-numbered elements, as the odd protons and possibly neutrons would hinder decay by spontaneous fission and result in longer decay chains.[59][65]
The first report of element 113 was in August 2003, when it was identified as an alpha decay product of element 115. Element 115 had been produced by bombarding a target of americium-243 with calcium-48 projectiles. The JINR–LLNL collaboration published its results in February 2004:[65]
- 243
95Am
+ 48
20Ca
→ 291115* → 288115 + 3
n
→ 284113 +
α - 243
95Am
+ 48
20Ca
→ 291115* → 287115 + 4
n
→ 283113 +
α
Four further alpha decays were observed, ending with the spontaneous fission of isotopes of element 105, dubnium.[65]
Riken
[edit]While the JINR–LLNL collaboration had been studying fusion reactions with 48Ca, a team of Japanese scientists at the Riken Nishina Center for Accelerator-Based Science in Wakō, Japan, led by Kōsuke Morita had been studying cold fusion reactions. Morita had previously studied the synthesis of superheavy elements at the JINR before starting his own team at Riken. In 2001, his team confirmed the GSI's discoveries of elements 108, 110, 111, and 112. They then made a new attempt on element 113, using the same 209Bi + 70Zn reaction that the GSI had attempted unsuccessfully in 1998. Despite the much lower yield expected than for the JINR's hot fusion technique with calcium-48, the Riken team chose to use cold fusion as the synthesised isotopes would alpha decay to known daughter nuclides and make the discovery much more certain, and would not require the use of radioactive targets.[66] In particular, the isotope 278113 expected to be produced in this reaction would decay to the known 266Bh, which had been synthesised in 2000 by a team at the Lawrence Berkeley National Laboratory (LBNL) in Berkeley.[67]
The bombardment of 209Bi with 70Zn at Riken began in September 2003.[68] The team detected a single atom of 278113 in July 2004 and published their results that September:[69]
The Riken team observed four alpha decays from 278113, creating a decay chain passing through 274Rg, 270Mt, and 266Bh before terminating with the spontaneous fission of 262Db.[69] The decay data they observed for the alpha decay of 266Bh matched the 2000 data, lending support for their claim. Spontaneous fission of its daughter 262Db had not been previously known; the American team had observed only alpha decay from this nuclide.[67]
Road to confirmation
[edit]When the discovery of a new element is claimed, the Joint Working Party (JWP) of the International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Pure and Applied Physics (IUPAP) assembles to examine the claims according to their criteria for the discovery of a new element, and decides scientific priority and naming rights for the elements. According to the JWP criteria, a discovery must demonstrate that the element has an atomic number different from all previously observed values. It should also preferably be repeated by other laboratories, although this requirement has been waived where the data is of very high quality. Such a demonstration must establish properties, either physical or chemical, of the new element and establish that they are those of a previously unknown element. The main techniques used to demonstrate atomic number are cross-reactions (creating claimed nuclides as parents or daughters of other nuclides produced by a different reaction) and anchoring decay chains to known daughter nuclides. For the JWP, priority in confirmation takes precedence over the date of the original claim. Both teams set out to confirm their results by these methods.[70]

2004–2008
[edit]In June 2004 and again in December 2005, the JINR–LLNL collaboration strengthened their claim for the discovery of element 113 by conducting chemical experiments on 268Db, the final decay product of 288115. This was valuable as none of the nuclides in this decay chain were previously known, so that their claim was not supported by any previous experimental data, and chemical experimentation would strengthen the case for their claim, since the chemistry of dubnium is known. 268Db was successfully identified by extracting the final decay products, measuring spontaneous fission (SF) activities and using chemical identification techniques to confirm that they behave like a group 5 element (dubnium is known to be in group 5).[1][71] Both the half-life and decay mode were confirmed for the proposed 268Db which lends support to the assignment of the parent and daughter nuclei to elements 115 and 113 respectively.[71][72] Further experiments at the JINR in 2005 confirmed the observed decay data.[67]
In November and December 2004, the Riken team studied the 205Tl + 70Zn reaction, aiming the zinc beam onto a thallium rather than a bismuth target, in an effort to directly produce 274Rg in a cross-bombardment as it is the immediate daughter of 278113. The reaction was unsuccessful, as the thallium target was physically weak compared to the more commonly used lead and bismuth targets, and it deteriorated significantly and became non-uniform in thickness. The reasons for this weakness are unknown, given that thallium has a higher melting point than bismuth.[73] The Riken team then repeated the original 209Bi + 70Zn reaction and produced a second atom of 278113 in April 2005, with a decay chain that again terminated with the spontaneous fission of 262Db. The decay data were slightly different from those of the first chain: this could have been because an alpha particle escaped from the detector without depositing its full energy, or because some of the intermediate decay products were formed in metastable isomeric states.[67]
In 2006, a team at the Heavy Ion Research Facility in Lanzhou, China, investigated the 243Am + 26Mg reaction, producing four atoms of 266Bh. All four chains started with an alpha decay to 262Db; three chains ended there with spontaneous fission, as in the 278113 chains observed at Riken, while the remaining one continued via another alpha decay to 258Lr, as in the 266Bh chains observed at LBNL.[70]
In June 2006, the JINR–LLNL collaboration claimed to have synthesised a new isotope of element 113 directly by bombarding a neptunium-237 target with accelerated calcium-48 nuclei:
Two atoms of 282113 were detected. The aim of this experiment had been to synthesise the isotopes 281113 and 282113 that would fill in the gap between isotopes produced via hot fusion (283113 and 284113) and cold fusion (278113). After five alpha decays, these nuclides would reach known isotopes of lawrencium, assuming that the decay chains were not terminated prematurely by spontaneous fission. The first decay chain ended in fission after four alpha decays, presumably originating from 266Db or its electron-capture daughter 266Rf. Spontaneous fission was not observed in the second chain even after four alpha decays. A fifth alpha decay in each chain could have been missed, since 266Db can theoretically undergo alpha decay, in which case the first decay chain would have ended at the known 262Lr or 262No and the second might have continued to the known long-lived 258Md, which has a half-life of 51.5 days, longer than the duration of the experiment: this would explain the lack of a spontaneous fission event in this chain. In the absence of direct detection of the long-lived alpha decays, these interpretations remain unconfirmed, and there is still no known link between any superheavy nuclides produced by hot fusion and the well-known main body of the chart of nuclides.[74]
2009–2015
[edit]The JWP published its report on elements 113–116 and 118 in 2011. It recognised the JINR–LLNL collaboration as having discovered elements 114 and 116, but did not accept either team's claim to element 113 and did not accept the JINR–LLNL claims to elements 115 and 118. The JINR–LLNL claim to elements 115 and 113 had been founded on chemical identification of their daughter dubnium, but the JWP objected that current theory could not distinguish between superheavy group 4 and group 5 elements by their chemical properties with enough confidence to allow this assignment.[67] The decay properties of all the nuclei in the decay chain of element 115 had not been previously characterised before the JINR experiments, a situation which the JWP generally considers "troublesome, but not necessarily exclusive", and with the small number of atoms produced with neither known daughters nor cross-reactions the JWP considered that their criteria had not been fulfilled.[67] The JWP did not accept the Riken team's claim either due to inconsistencies in the decay data, the small number of atoms of element 113 produced, and the lack of unambiguous anchors to known isotopes.[67]
In early 2009, the Riken team synthesised the decay product 266Bh directly in the 248Cm + 23Na reaction to establish its link with 278113 as a cross-bombardment. They also established the branched decay of 262Db, which sometimes underwent spontaneous fission and sometimes underwent the previously known alpha decay to 258Lr.[75][76]
In late 2009, the JINR–LLNL collaboration studied the 249Bk + 48Ca reaction in an effort to produce element 117, which would decay to elements 115 and 113 and bolster their claims in a cross-reaction. They were now joined by scientists from Oak Ridge National Laboratory (ORNL) and Vanderbilt University, both in Tennessee, United States,[59] who helped procure the rare and highly radioactive berkelium target necessary to complete the JINR's calcium-48 campaign to synthesise the heaviest elements on the periodic table.[59] Two isotopes of element 117 were synthesised, decaying to element 115 and then element 113:[77]
- 249
97Bk
+ 48
20Ca
→ 297117* → 294117 + 3
n
→ 290115 + α → 286113 + α - 249
97Bk
+ 48
20Ca
→ 297117* → 293117 + 4
n
→ 289115 + α → 285113 + α
The new isotopes 285113 and 286113 produced did not overlap with the previously claimed 282113, 283113, and 284113, so this reaction could not be used as a cross-bombardment to confirm the 2003 or 2006 claims.[70]
In March 2010, the Riken team again attempted to synthesise 274Rg directly through the 205Tl + 70Zn reaction with upgraded equipment; they failed again and abandoned this cross-bombardment route.[73]
After 450 more days of irradiation of bismuth with zinc projectiles, Riken produced and identified another 278113 atom in August 2012.[78] Although electricity prices had soared since the 2011 Tōhoku earthquake and tsunami, and Riken had ordered the shutdown of the accelerator programs to save money, Morita's team was permitted to continue with one experiment, and they chose their attempt to confirm their synthesis of element 113.[79] In this case, a series of six alpha decays was observed, leading to an isotope of mendelevium:
This decay chain differed from the previous observations at Riken mainly in the decay mode of 262Db, which was previously observed to undergo spontaneous fission, but in this case instead alpha decayed; the alpha decay of 262Db to 258Lr is well-known. The team calculated the probability of accidental coincidence to be 10−28, or totally negligible.[78] The resulting 254Md atom then underwent electron capture to 254Fm, which underwent the seventh alpha decay in the chain to the long-lived 250Cf, which has a half-life of around thirteen years.[80]
The 249Bk + 48Ca experiment was repeated at the JINR in 2012 and 2013 with consistent results, and again at the GSI in 2014.[70] In August 2013, a team of researchers at Lund University in Lund, Sweden, and at the GSI announced that they had repeated the 2003 243Am + 48Ca experiment, confirming the findings of the JINR–LLNL collaboration.[68][81] The same year, the 2003 experiment had been repeated at the JINR, now also creating the isotope 289115 that could serve as a cross-bombardment for confirming their discovery of the element 117 isotope 293117, as well as its daughter 285113 as part of its decay chain.[70] Confirmation of 288115 and its daughters was published by the team at the LBNL in August 2015.[82]
Approval of discoveries
[edit]In December 2015, the conclusions of a new JWP report were published by IUPAC in a press release, in which element 113 was awarded to Riken; elements 115, 117, and 118 were awarded to the collaborations involving the JINR.[83] A joint 2016 announcement by IUPAC and IUPAP had been scheduled to coincide with the publication of the JWP reports, but IUPAC alone decided on an early release because the news of Riken being awarded credit for element 113 had been leaked to Japanese newspapers.[84] For the first time in history, a team of Asian physicists would name a new element.[83] The JINR considered the awarding of element 113 to Riken unexpected, citing their own 2003 production of elements 115 and 113, and pointing to the precedents of elements 103, 104, and 105 where IUPAC had awarded joint credit to the JINR and LBNL. They stated that they respected IUPAC's decision, but reserved determination of their position for the official publication of the JWP reports.[85]
The full JWP reports were published on 21 January 2016. The JWP recognised the discovery of element 113, assigning priority to Riken. They noted that while the individual decay energies of each nuclide in the decay chain of 278113 were inconsistent, their sum was now confirmed to be consistent, strongly suggesting that the initial and final states in 278113 and its daughter 262Db were the same for all three events. The decay of 262Db to 258Lr and 254Md was previously known, firmly anchoring the decay chain of 278113 to known regions of the chart of nuclides. The JWP considered that the JINR–LLNL collaborations of 2004 and 2007, producing element 113 as the daughter of element 115, did not meet the discovery criteria as they had not convincingly determined the atomic numbers of their nuclides through cross-bombardments, which were considered necessary since their decay chains were not anchored to previously known nuclides. They also considered that the previous JWP's concerns over their chemical identification of the dubnium daughter had not been adequately addressed. The JWP recognised the JINR–LLNL–ORNL–Vanderbilt collaboration of 2010 as having discovered elements 117 and 115, and accepted that element 113 had been produced as their daughter, but did not give this work shared credit.[70][73][86]
After the publication of the JWP reports, Sergey Dimitriev, the lab director of the Flerov lab at the JINR where the discoveries were made, remarked that he was happy with IUPAC's decision, mentioning the time Riken spent on their experiment and their good relations with Morita, who had learnt the basics of synthesising superheavy elements at the JINR.[59][85]
The sum argument advanced by the JWP in the approval of the discovery of element 113 was later criticised in a May 2016 study from Lund University and the GSI, as it is only valid if no gamma decay or internal conversion takes place along the decay chain, which is not likely for odd nuclei, and the uncertainty of the alpha decay energies measured in the 278113 decay chain was not small enough to rule out this possibility. If this is the case, similarity in lifetimes of intermediate daughters becomes a meaningless argument, as different isomers of the same nuclide can have different half-lives: for example, the ground state of 180Ta has a half-life of hours, but an excited state 180mTa has never been observed to decay. This study found reason to doubt and criticise the IUPAC approval of the discoveries of elements 115 and 117, but the data from Riken for element 113 was found to be congruent, and the data from the JINR team for elements 115 and 113 to probably be so, thus endorsing the IUPAC approval of the discovery of element 113.[87][88] Two members of the JINR team published a journal article rebutting these criticisms against the congruence of their data on elements 113, 115, and 117 in June 2017.[89]
Naming
[edit]
Using Mendeleev's nomenclature for unnamed and undiscovered elements, nihonium would be known as eka-thallium. In 1979, IUPAC published recommendations according to which the element was to be called ununtrium (with the corresponding symbol of Uut),[90] a systematic element name as a placeholder, until the discovery of the element is confirmed and a name is decided on. The recommendations were widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, but were mostly ignored among scientists in the field, who called it "element 113", with the symbol of E113, (113), or even simply 113.[1]
Before the JWP recognition of their priority, the Japanese team had unofficially suggested various names: japonium, after their home country;[91] nishinanium, after Japanese physicist Yoshio Nishina, the "founding father of modern physics research in Japan";[92] and rikenium, after the institute.[91] After the recognition, the Riken team gathered in February 2016 to decide on a name. Morita expressed his desire for the name to honour the fact that element 113 had been discovered in Japan. Japonium was considered, making the connection to Japan easy to identify for non-Japanese, but it was rejected as Jap is considered an ethnic slur. The name nihonium was chosen after an hour of deliberation: it comes from nihon (日本), one of the two Japanese pronunciations for the name of Japan.[93] The discoverers also intended to reference the support of their research by the Japanese people (Riken being almost entirely government-funded),[94] recover lost pride and trust in science among those who were affected by the Fukushima Daiichi nuclear disaster,[95] and honour Japanese chemist Masataka Ogawa's 1908 discovery of rhenium, which he named "nipponium" with symbol Np after the other Japanese pronunciation of Japan's name.[86] As Ogawa's claim had not been accepted, the name "nipponium" could not be reused for a new element, and its symbol Np had since been used for neptunium.[m] In March 2016, Morita proposed the name "nihonium" to IUPAC, with the symbol Nh.[86] The naming realised what had been a national dream in Japanese science ever since Ogawa's claim.[79]
The former president of IUPAP, Cecilia Jarlskog, complained at the Nobel Symposium on Superheavy Elements in Bäckaskog Castle, Sweden, in June 2016 about the lack of openness involved in the process of approving new elements, and stated that she believed that the JWP's work was flawed and should be redone by a new JWP. A survey of physicists determined that many felt that the Lund–GSI 2016 criticisms of the JWP report were well-founded, but it was also generally thought that the conclusions would hold up if the work was redone. Thus the new president, Bruce McKellar, ruled that the proposed names should be released in a joint IUPAP–IUPAC press release.[84] IUPAC and IUPAP publicised the proposal of nihonium that June,[95] and set a five-month term to collect comments, after which the name would be formally established at a conference.[98][99] The name was officially approved on 28 November 2016.[100] The naming ceremony for the new element was held in Tokyo, Japan, on 14 March 2017, with Naruhito, then the Crown Prince of Japan, in attendance.[101]
Isotopes
[edit]| Isotope | Half-life[n] | Decay mode |
Discovery year |
Discovery reaction | |
|---|---|---|---|---|---|
| Value | ref | ||||
| 278Nh | 2.0 ms | [7] | α | 2004 | 209Bi(70Zn,n) |
| 282Nh | 61 ms | [102] | α | 2006 | 237Np(48Ca,3n) |
| 283Nh | 123 ms | [102] | α | 2004 | 287Mc(—,α) |
| 284Nh | 0.90 s | [102] | α, EC | 2004 | 288Mc(—,α) |
| 285Nh | 2.1 s | [102] | α, SF | 2010 | 289Mc(—,α) |
| 286Nh | 9.5 s | [7] | α | 2010 | 290Mc(—,α) |
| 287Nh[o] | 5.5 s | [8] | α | 1999 | 287Fl(e−,νe) |
| 290Nh[o] | 2 s | [9] | α | 1998 | 290Fl(e−,νe) |
Nihonium has no stable or naturally occurring isotopes. Several radioactive isotopes have been synthesised in the laboratory, either by fusing two atoms or by observing the decay of heavier elements. Eight different isotopes of nihonium have been reported with atomic masses 278, 282–287, and 290 (287Nh and 290Nh are unconfirmed); they all decay through alpha decay to isotopes of roentgenium.[103] There have been indications that nihonium-284 can also decay by electron capture to copernicium-284, though estimates of the partial half-life for this branch vary strongly by model.[104] A spontaneous fission branch of nihonium-285 has also been reported.[102]
Stability and half-lives
[edit]
The stability of nuclei quickly decreases with the increase in atomic number after curium, element 96, whose half-life is over ten thousand times longer than that of any subsequent element. All isotopes with an atomic number above 101 undergo radioactive decay with half-lives of less than 30 hours: this is because of the ever-increasing Coulomb repulsion of protons, so that the strong nuclear force cannot hold the nucleus together against spontaneous fission for long. Calculations suggest that in the absence of other stabilising factors, elements with more than 103 protons should not exist. Researchers in the 1960s suggested that the closed nuclear shells around 114 protons and 184 neutrons should counteract this instability, and create an "island of stability" containing nuclides with half-lives reaching thousands or millions of years. The existence of the island is still unproven, but the existence of the superheavy elements (including nihonium) confirms that the stabilising effect is real, and in general the known superheavy nuclides become longer-lived as they approach the predicted location of the island.[105][106]
All nihonium isotopes are unstable and radioactive; the heavier nihonium isotopes are more stable than the lighter ones, as they are closer to the centre of the island. The most stable known nihonium isotope, 286Nh, is also the heaviest; it has a half-life of 8 seconds. The isotope 285Nh, as well as the unconfirmed 287Nh and 290Nh, have also been reported to have half-lives of over a second. The isotopes 284Nh and 283Nh have half-lives of 0.90 and 0.12 seconds respectively. The remaining two isotopes have half-lives between 0.1 and 100 milliseconds: 282Nh has a half-life of 61 milliseconds, and 278Nh, the lightest known nihonium isotope, is also the shortest-lived, with a half-life of 2.0 milliseconds. This rapid increase in the half-lives near the closed neutron shell at N = 184 is seen in roentgenium, copernicium, and nihonium (elements 111 through 113), where each extra neutron so far multiplies the half-life by a factor of 5 to 20.[106][107]
The unknown isotopes in the gap between 278Nh and 282Nh are too heavy to be produced by cold fusion and too light to be produced by hot fusion. The missing 280Nh and 281Nh may be populated as daughters of 284Mc and 285Mc, producible in the 241Am+48Ca reaction, but this has not yet been attempted.[108] Of particular interest is 281Nh, as it is the expected great-granddaughter of 293119, a possible product of the 243Am+54Cr reaction.[109] Production of 282Mc and 283Mc is possible in the 243Am+44Ca reaction (though it has a lower cross-section), and their daughters would be 278Nh (known) and 279Nh.[108] The heavier isotopes 287Nh through 290Nh might be synthesised using charged-particle evaporation, using the 242Pu+48Ca and 244Pu+48Ca reactions where one proton and some neutrons are evaporated.[110][111]
Predicted properties
[edit]Very few properties of nihonium or its compounds have been measured; this is due to its extremely limited and expensive production[112] and the fact it decays very quickly. Properties of nihonium mostly remain unknown and only predictions are available.
Physical and atomic
[edit]
Nihonium is the first member of the 7p series of elements and the heaviest group 13 element on the periodic table, below boron, aluminium, gallium, indium, and thallium. All the group 13 elements except boron are metals, and nihonium is expected to follow suit. Nihonium is predicted to show many differences from its lighter homologues. The major reason for this is the spin–orbit (SO) interaction, which is especially strong for the superheavy elements, because their electrons move much faster than in lighter atoms, at velocities close to the speed of light.[114]: 63 In relation to nihonium atoms, it lowers the 7s and the 7p electron energy levels (stabilising those electrons), but two of the 7p electron energy levels are stabilised more than the other four.[115] The stabilisation of the 7s electrons is called the inert pair effect, and the separation of the 7p subshell into the more and less stabilised parts is called subshell splitting. Computational chemists see the split as a change of the second, azimuthal quantum number l, from 1 to 1/2 and 3/2 for the more and less stabilised parts of the 7p subshell, respectively.[114]: 63 The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. For theoretical purposes, the valence electron configuration may be represented to reflect the 7p subshell split as 7s2 7p1/21.[1] The first ionisation energy of nihonium is expected to be 7.306 eV, the highest among the metals of group 13.[1] Similar subshell splitting should exist for the 6d electron levels, with four being 6d3/2 and six being 6d5/2. Both these levels are raised to be close in energy to the 7s ones, high enough to possibly be chemically active. This would allow for the possibility of exotic nihonium compounds without lighter group 13 analogues.[115]
Periodic trends would predict nihonium to have an atomic radius larger than that of thallium due to it being one period further down the periodic table, but calculations suggest nihonium has an atomic radius of about 170 pm, the same as that of thallium, due to the relativistic stabilisation and contraction of its 7s and 7p1/2 orbitals. Thus, nihonium is expected to be much denser than thallium, with a predicted density of about 16 to 18 g/cm3 compared to thallium's 11.85 g/cm3, since nihonium atoms are heavier than thallium atoms but have the same volume.[1][113] Bulk nihonium is expected to have a hexagonal close-packed crystal structure, like thallium.[5] The melting and boiling points of nihonium have been predicted to be 430 °C and 1100 °C respectively, exceeding the values for indium and thallium, following periodic trends.[1][2] Nihonium should have a bulk modulus of 20.8 GPa, about half that of thallium (43 GPa).[6]
Chemical
[edit]The chemistry of nihonium is expected to be very different from that of thallium. This difference stems from the spin–orbit splitting of the 7p shell, which results in nihonium being between two relatively inert closed-shell elements (copernicium and flerovium).[116] Nihonium is expected to be less reactive than thallium, because of the greater stabilisation and resultant chemical inactivity of the 7s subshell in nihonium compared to the 6s subshell in thallium.[4] The standard electrode potential for the Nh+/Nh couple is predicted to be 0.6 V. Nihonium should be a rather noble metal.[4]
The metallic group 13 elements are typically found in two oxidation states: +1 and +3. The former results from the involvement of only the single p electron in bonding, and the latter results in the involvement of all three valence electrons, two in the s-subshell and one in the p-subshell. Going down the group, bond energies decrease and the +3 state becomes less stable, as the energy released in forming two additional bonds and attaining the +3 state is not always enough to outweigh the energy needed to involve the s-electrons. Hence, for aluminium and gallium +3 is the most stable state, but +1 gains importance for indium and by thallium it becomes more stable than the +3 state. Nihonium is expected to continue this trend and have +1 as its most stable oxidation state.[1]
The simplest possible nihonium compound is the monohydride, NhH. The bonding is provided by the 7p1/2 electron of nihonium and the 1s electron of hydrogen. The SO interaction causes the binding energy of nihonium monohydride to be reduced by about 1 eV[1] and the nihonium–hydrogen bond length to decrease as the bonding 7p1/2 orbital is relativistically contracted. This is unique among the 7p element monohydrides; all the others have relativistic expansion of the bond length instead of contraction.[117] Another effect of the SO interaction is that the Nh–H bond is expected to have significant pi bonding character (side-on orbital overlap), unlike the almost pure sigma bonding (head-on orbital overlap) in thallium monohydride (TlH).[118] The analogous monofluoride (NhF) should also exist.[113] Nihonium(I) is predicted to be more similar to silver(I) than thallium(I):[1] the Nh+ ion is expected to more willingly bind anions, so that NhCl should be quite soluble in excess hydrochloric acid or ammonia; TlCl is not. In contrast to Tl+, which forms the strongly basic hydroxide (TlOH) in solution, the Nh+ cation should instead hydrolyse all the way to the amphoteric oxide Nh2O, which would be soluble in aqueous ammonia and weakly soluble in water.[4]
The adsorption behaviour of nihonium on gold surfaces in thermochromatographical experiments is expected to be closer to that of astatine than that of thallium. The destabilisation of the 7p3/2 subshell effectively leads to a valence shell closing at the 7s2 7p2 configuration rather than the expected 7s2 7p6 configuration with its stable octet. As such, nihonium, like astatine, can be considered to be one p-electron short of a closed valence shell. Hence, even though nihonium is in group 13, it has several properties similar to the group 17 elements. (Tennessine in group 17 has some group-13-like properties, as it has three valence electrons outside the 7s2 7p2 closed shell.[119]) Nihonium is expected to be able to gain an electron to attain this closed-shell configuration, forming the −1 oxidation state like the halogens (fluorine, chlorine, bromine, iodine, and astatine). This state should be more stable than it is for thallium as the SO splitting of the 7p subshell is greater than that for the 6p subshell.[114]: 63 Nihonium should be the most electronegative of the metallic group 13 elements,[1] even more electronegative than tennessine, the period 7 congener of the halogens: in the compound NhTs, the negative charge is expected to be on the nihonium atom rather than the tennessine atom.[113] The −1 oxidation should be more stable for nihonium than for tennessine.[1][120] The electron affinity of nihonium is calculated to be around 0.68 eV, higher than thallium's at 0.4 eV; tennessine's is expected to be 1.8 eV, the lowest in its group.[1] It is theoretically predicted that nihonium should have an enthalpy of sublimation around 150 kJ/mol and an enthalpy of adsorption on a gold surface around −159 kJ/mol.[121]
3 has a trigonal structure.
Significant 6d involvement is expected in the Nh–Au bond, although it is expected to be more unstable than the Tl–Au bond and entirely due to magnetic interactions. This raises the possibility of some transition metal character for nihonium.[116] On the basis of the small energy gap between the 6d and 7s electrons, the higher oxidation states +3 and +5 have been suggested for nihonium.[1][4] Some simple compounds with nihonium in the +3 oxidation state would be the trihydride (NhH3), trifluoride (NhF3), and trichloride (NhCl3). These molecules are predicted to be T-shaped and not trigonal planar as their boron analogues are:[p] this is due to the influence of the 6d5/2 electrons on the bonding.[118][q] The heavier nihonium tribromide (NhBr3) and triiodide (NhI3) are trigonal planar due to the increased steric repulsion between the peripheral atoms; accordingly, they do not show significant 6d involvement in their bonding, though the large 7s–7p energy gap means that they show reduced sp2 hybridisation compared to their boron analogues.[118]
The bonding in the lighter NhX3 molecules can be considered as that of a linear NhX+
2 species (similar to HgF2 or AuF−
2) with an additional Nh–X bond involving the 7p orbital of nihonium perpendicular to the other two ligands. These compounds are all expected to be highly unstable towards the loss of an X2 molecule and reduction to nihonium(I):[118]
- NhX3 → NhX + X2
Nihonium thus continues the trend down group 13 of reduced stability of the +3 oxidation state, as all five of these compounds have lower reaction energies than the unknown thallium(III) iodide.[r] The +3 state is stabilised for thallium in anionic complexes such as TlI−
4, and the presence of a possible vacant coordination site on the lighter T-shaped nihonium trihalides is expected to allow a similar stabilisation of NhF−
4 and perhaps NhCl−
4.[118]
The +5 oxidation state is unknown for all lighter group 13 elements: calculations predict that nihonium pentahydride (NhH5) and pentafluoride (NhF5) should have a square pyramidal molecular geometry, but also that both would be highly thermodynamically unstable to loss of an X2 molecule and reduction to nihonium(III). Again, some stabilisation is expected for anionic complexes, such as NhF−
6. The structures of the nihonium trifluoride and pentafluoride molecules are the same as those for chlorine trifluoride and pentafluoride.[118]
Experimental chemistry
[edit]The chemical characteristics of nihonium have yet to be determined unambiguously.[121][126] The isotopes 284Nh, 285Nh, and 286Nh have half-lives long enough for chemical investigation.[121] From 2010 to 2012, some preliminary chemical experiments were performed at the JINR to determine the volatility of nihonium. The isotope 284Nh was investigated, made as the daughter of 288Mc produced in the 243Am+48Ca reaction. The nihonium atoms were synthesised in a recoil chamber and then carried along polytetrafluoroethylene (PTFE) capillaries at 70 °C by a carrier gas to the gold-covered detectors. About ten to twenty atoms of 284Nh were produced, but none of these atoms were registered by the detectors, suggesting either that nihonium was similar in volatility to the noble gases (and thus diffused away too quickly to be detected) or, more plausibly, that pure nihonium was not very volatile and thus could not efficiently pass through the PTFE capillaries.[121] Formation of the hydroxide NhOH should ease the transport, as nihonium hydroxide is expected to be more volatile than elemental nihonium, and this reaction could be facilitated by adding more water vapour into the carrier gas. It seems likely that this formation is not kinetically favoured, so the longer-lived isotopes 285Nh and 286Nh were considered more desirable for future experiments.[121][127]
A 2017 experiment at the JINR, producing 284Nh and 285Nh via the 243Am+48Ca reaction as the daughters of 288Mc and 289Mc, avoided this problem by removing the quartz surface, using only PTFE. No nihonium atoms were observed after chemical separation, implying an unexpectedly large retention of nihonium atoms on PTFE surfaces. This experimental result for the interaction limit of nihonium atoms with a PTFE surface (−ΔHPTFE
ads(Nh) > 45 kJ/mol) disagrees significantly with previous theory, which expected a lower value of 14.00 kJ/mol. This suggests that the nihonium species involved in the previous experiment was likely not elemental nihonium but rather nihonium hydroxide, and that high-temperature techniques such as vacuum chromatography would be necessary to further probe the behaviour of elemental nihonium.[39] Bromine saturated with boron tribromide has been suggested as a carrier gas for experiments on nihonium chemistry; this oxidises nihonium's lighter congener thallium to thallium(III), providing an avenue to investigate the oxidation states of nihonium, similar to earlier experiments done on the bromides of group 5 elements, including the superheavy dubnium.[128]
Notes
[edit]- ^ In nuclear physics, an element is called heavy if its atomic number is high; lead (element 82) is one example of such a heavy element. The term "superheavy elements" typically refers to elements with atomic number greater than 103 (although there are other definitions, such as atomic number greater than 100[13] or 112;[14] sometimes, the term is presented an equivalent to the term "transactinide", which puts an upper limit before the beginning of the hypothetical superactinide series).[15] Terms "heavy isotopes" (of a given element) and "heavy nuclei" mean what could be understood in the common language—isotopes of high mass (for the given element) and nuclei of high mass, respectively.
- ^ In 2009, a team at the JINR led by Oganessian published results of their attempt to create hassium in a symmetric 136Xe + 136Xe reaction. They failed to observe a single atom in such a reaction, putting the upper limit on the cross section, the measure of probability of a nuclear reaction, as 2.5 pb.[16] In comparison, the reaction that resulted in hassium discovery, 208Pb + 58Fe, had a cross section of ~20 pb (more specifically, 19+19
-11 pb), as estimated by the discoverers.[17] - ^ The amount of energy applied to the beam particle to accelerate it can also influence the value of cross section. For example, in the 28
14Si
+ 1
0n
→ 28
13Al
+ 1
1p
reaction, cross section changes smoothly from 370 mb at 12.3 MeV to 160 mb at 18.3 MeV, with a broad peak at 13.5 MeV with the maximum value of 380 mb.[21] - ^ This figure also marks the generally accepted upper limit for lifetime of a compound nucleus.[26]
- ^ This separation is based on that the resulting nuclei move past the target more slowly then the unreacted beam nuclei. The separator contains electric and magnetic fields whose effects on a moving particle cancel out for a specific velocity of a particle.[28] Such separation can also be aided by a time-of-flight measurement and a recoil energy measurement; a combination of the two may allow to estimate the mass of a nucleus.[29]
- ^ Not all decay modes are caused by electrostatic repulsion. For example, beta decay is caused by the weak interaction.[36]
- ^ It was already known by the 1960s that ground states of nuclei differed in energy and shape as well as that certain magic numbers of nucleons corresponded to greater stability of a nucleus. However, it was assumed that there was no nuclear structure in superheavy nuclei as they were too deformed to form one.[41]
- ^ Since mass of a nucleus is not measured directly but is rather calculated from that of another nucleus, such measurement is called indirect. Direct measurements are also possible, but for the most part they have remained unavailable for superheavy nuclei.[46] The first direct measurement of mass of a superheavy nucleus was reported in 2018 at LBNL.[47] Mass was determined from the location of a nucleus after the transfer (the location helps determine its trajectory, which is linked to the mass-to-charge ratio of the nucleus, since the transfer was done in presence of a magnet).[48]
- ^ If the decay occurred in a vacuum, then since total momentum of an isolated system before and after the decay must be preserved, the daughter nucleus would also receive a small velocity. The ratio of the two velocities, and accordingly the ratio of the kinetic energies, would thus be inverse to the ratio of the two masses. The decay energy equals the sum of the known kinetic energy of the alpha particle and that of the daughter nucleus (an exact fraction of the former).[37] The calculations hold for an experiment as well, but the difference is that the nucleus does not move after the decay because it is tied to the detector.
- ^ Spontaneous fission was discovered by Soviet physicist Georgy Flerov,[49] a leading scientist at JINR, and thus it was a "hobbyhorse" for the facility.[50] In contrast, the LBL scientists believed fission information was not sufficient for a claim of synthesis of an element. They believed spontaneous fission had not been studied enough to use it for identification of a new element, since there was a difficulty of establishing that a compound nucleus had only ejected neutrons and not charged particles like protons or alpha particles.[26] They thus preferred to link new isotopes to the already known ones by successive alpha decays.[49]
- ^ For instance, element 102 was mistakenly identified in 1957 at the Nobel Institute of Physics in Stockholm, Stockholm County, Sweden.[51] There were no earlier definitive claims of creation of this element, and the element was assigned a name by its Swedish, American, and British discoverers, nobelium. It was later shown that the identification was incorrect.[52] The following year, RL was unable to reproduce the Swedish results and announced instead their synthesis of the element; that claim was also disproved later.[52] JINR insisted that they were the first to create the element and suggested a name of their own for the new element, joliotium;[53] the Soviet name was also not accepted (JINR later referred to the naming of the element 102 as "hasty").[54] This name was proposed to IUPAC in a written response to their ruling on priority of discovery claims of elements, signed 29 September 1992.[54] The name "nobelium" remained unchanged on account of its widespread usage.[55]
- ^ Transactinide elements, such as nihonium, are produced by nuclear fusion. These fusion reactions can be divided into "hot" and "cold" fusion, depending on the excitation energy of the compound nucleus produced. "Cold fusion" in the context of superheavy element synthesis is a distinct concept from the idea that nuclear fusion can be achieved under room temperature conditions.[56] In hot fusion reactions, light, high-energy projectiles are accelerated towards heavy targets (actinides), creating compound nuclei at high excitation energy (~40–50 MeV) that may fission, or alternatively emit several (3 to 5) neutrons.[57] Cold fusion reactions use heavier projectiles, typically from the fourth period, and lighter targets, usually lead and bismuth. The fused nuclei produced have a relatively low excitation energy (~10–20 MeV), which decreases the probability that they will undergo fission reactions. As the fused nuclei cool to the ground state, they emit only one or two neutrons. Hot fusion produces more neutron-rich products because actinides have the highest neutron-to-proton ratios of any elements, and is currently the only method to produce the superheavy elements from flerovium (element 114) onwards.[58]
- ^ Neptunium had been first reported at Riken by Nishina and Kenjiro Kimura in 1940, who did not get naming rights because they could not chemically separate and identify their discovery.[96][97]
- ^ Different sources give different values for half-lives; the most recently published values are listed.
- ^ a b This isotope is unconfirmed
- ^ Among the stable group 13 elements, only boron forms monomeric halides at standard conditions; those of aluminium, gallium, indium, and thallium form ionic lattice structures or (in a few cases) dimerise.[122][123]
- ^ The opposite effect is expected for the superheavy member of group 17, tennessine, due to the relativistic stabilisation of the 7p1/2 orbital: thus IF3 is T-shaped, but TsF3 is expected to be trigonal planar.[124]
- ^ The compound with stoichiometry TlI3 is a thallium(I) compound involving the triiodide anion, I−
3.[125]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 978-1-4020-3555-5.
- ^ a b c Seaborg, Glenn T. (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 16 March 2010.
- ^ a b c Bonchev, Danail; Kamenska, Verginia (1981). "Predicting the Properties of the 113–120 Transactinide Elements". Journal of Physical Chemistry. 85 (9): 1177–1186. doi:10.1021/j150609a021.
- ^ a b c d e f g h i Fricke, Burkhard (1975). "Superheavy elements: a prediction of their chemical and physical properties". Recent Impact of Physics on Inorganic Chemistry. Structure and Bonding. 21: 89–144. doi:10.1007/BFb0116498. ISBN 978-3-540-07109-9. Retrieved 4 October 2013.
- ^ a b Keller, O. L. Jr.; Burnett, J. L.; Carlson, T. A.; Nestor, C. W. Jr. (1969). "Predicted Properties of the Super Heavy Elements. I. Elements 113 and 114, Eka-Thallium and Eka-Lead". The Journal of Physical Chemistry. 74 (5): 1127−1134. doi:10.1021/j100700a029.
- ^ a b Atarah, Samuel A.; Egblewogbe, Martin N. H.; Hagoss, Gebreyesus G. (2020). "First principle study of the structural and electronic properties of Nihonium". MRS Advances: 1–9. doi:10.1557/adv.2020.159.
- ^ a b c Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ a b c Hofmann, S.; Heinz, S.; Mann, R.; Maurer, J.; Münzenberg, G.; Antalic, S.; Barth, W.; et al. (2016). "Remarks on the Fission Barriers of SHN and Search for Element 120". In Peninozhkevich, Yu. E.; Sobolev, Yu. G. (eds.). Exotic Nuclei: EXON-2016 Proceedings of the International Symposium on Exotic Nuclei. Exotic Nuclei. pp. 155–164. ISBN 9789813226555.
- ^ a b c Hofmann, S.; Heinz, S.; Mann, R.; Maurer, J.; Münzenberg, G.; Antalic, S.; Barth, W.; et al. (2016). "Review of even element super-heavy nuclei and search for element 120". The European Physics Journal A. 2016 (52). doi:10.1140/epja/i2016-16180-4.
- ^ "WebElements Periodic Table » Nihonium » the essentials". www.webelements.com. Retrieved 8 June 2024.
- ^ "Nihonium | Nh (Element) - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 8 June 2024.
- ^ "Nihonium (Nh) | AMERICAN ELEMENTS ®". American Elements: The Materials Science Company. Retrieved 24 April 2024.
- ^ Krämer, K. (2016). "Explainer: superheavy elements". Chemistry World. Retrieved 15 March 2020.
- ^ "Discovery of Elements 113 and 115". Lawrence Livermore National Laboratory. Archived from the original on 11 September 2015. Retrieved 15 March 2020.
- ^ Eliav, E.; Kaldor, U.; Borschevsky, A. (2018). "Electronic Structure of the Transactinide Atoms". In Scott, R. A. (ed.). Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons. pp. 1–16. doi:10.1002/9781119951438.eibc2632. ISBN 978-1-119-95143-8. S2CID 127060181.
- ^ Oganessian, Yu. Ts.; Dmitriev, S. N.; Yeremin, A. V.; et al. (2009). "Attempt to produce the isotopes of element 108 in the fusion reaction 136Xe + 136Xe". Physical Review C. 79 (2): 024608. doi:10.1103/PhysRevC.79.024608. ISSN 0556-2813.
- ^ Münzenberg, G.; Armbruster, P.; Folger, H.; et al. (1984). "The identification of element 108" (PDF). Zeitschrift für Physik A. 317 (2): 235–236. Bibcode:1984ZPhyA.317..235M. doi:10.1007/BF01421260. S2CID 123288075. Archived from the original (PDF) on 7 June 2015. Retrieved 20 October 2012.
- ^ Subramanian, S. (28 August 2019). "Making New Elements Doesn't Pay. Just Ask This Berkeley Scientist". Bloomberg Businessweek. Retrieved 18 January 2020.
- ^ a b c d e f Ivanov, D. (2019). "Сверхтяжелые шаги в неизвестное" [Superheavy steps into the unknown]. nplus1.ru (in Russian). Retrieved 2 February 2020.
- ^ Hinde, D. (2017). "Something new and superheavy at the periodic table". The Conversation. Retrieved 30 January 2020.
- ^ Kern, B. D.; Thompson, W. E.; Ferguson, J. M. (1959). "Cross sections for some (n, p) and (n, α) reactions". Nuclear Physics. 10: 226–234. Bibcode:1959NucPh..10..226K. doi:10.1016/0029-5582(59)90211-1.
- ^ Wakhle, A.; Simenel, C.; Hinde, D. J.; et al. (2015). Simenel, C.; Gomes, P. R. S.; Hinde, D. J.; et al. (eds.). "Comparing Experimental and Theoretical Quasifission Mass Angle Distributions". European Physical Journal Web of Conferences. 86: 00061. Bibcode:2015EPJWC..8600061W. doi:10.1051/epjconf/20158600061. hdl:1885/148847. ISSN 2100-014X.
- ^ "Nuclear Reactions" (PDF). pp. 7–8. Retrieved 27 January 2020. Published as Loveland, W. D.; Morrissey, D. J.; Seaborg, G. T. (2005). "Nuclear Reactions". Modern Nuclear Chemistry. John Wiley & Sons, Inc. pp. 249–297. doi:10.1002/0471768626.ch10. ISBN 978-0-471-76862-3.
- ^ a b Krása, A. (2010). "Neutron Sources for ADS". Faculty of Nuclear Sciences and Physical Engineering. Czech Technical University in Prague: 4–8. S2CID 28796927.
- ^ Wapstra, A. H. (1991). "Criteria that must be satisfied for the discovery of a new chemical element to be recognized" (PDF). Pure and Applied Chemistry. 63 (6): 883. doi:10.1351/pac199163060879. ISSN 1365-3075. S2CID 95737691.
- ^ a b Hyde, E. K.; Hoffman, D. C.; Keller, O. L. (1987). "A History and Analysis of the Discovery of Elements 104 and 105". Radiochimica Acta. 42 (2): 67–68. doi:10.1524/ract.1987.42.2.57. ISSN 2193-3405. S2CID 99193729.
- ^ a b c d Chemistry World (2016). "How to Make Superheavy Elements and Finish the Periodic Table [Video]". Scientific American. Retrieved 27 January 2020.
- ^ Hoffman, Ghiorso & Seaborg 2000, p. 334.
- ^ Hoffman, Ghiorso & Seaborg 2000, p. 335.
- ^ Zagrebaev, Karpov & Greiner 2013, p. 3.
- ^ Beiser 2003, p. 432.
- ^ a b Pauli, N. (2019). "Alpha decay" (PDF). Introductory Nuclear, Atomic and Molecular Physics (Nuclear Physics Part). Université libre de Bruxelles. Retrieved 16 February 2020.
- ^ a b c d e Pauli, N. (2019). "Nuclear fission" (PDF). Introductory Nuclear, Atomic and Molecular Physics (Nuclear Physics Part). Université libre de Bruxelles. Retrieved 16 February 2020.
- ^ Staszczak, A.; Baran, A.; Nazarewicz, W. (2013). "Spontaneous fission modes and lifetimes of superheavy elements in the nuclear density functional theory". Physical Review C. 87 (2): 024320–1. arXiv:1208.1215. Bibcode:2013PhRvC..87b4320S. doi:10.1103/physrevc.87.024320. ISSN 0556-2813.
- ^ Audi et al. 2017, pp. 030001-129–030001-138.
- ^ Beiser 2003, p. 439.
- ^ a b Beiser 2003, p. 433.
- ^ Audi et al. 2017, p. 030001-125.
- ^ a b Aksenov, N. V.; Steinegger, P.; Abdullin, F. Sh.; et al. (2017). "On the volatility of nihonium (Nh, Z = 113)". The European Physical Journal A. 53 (7): 158. Bibcode:2017EPJA...53..158A. doi:10.1140/epja/i2017-12348-8. ISSN 1434-6001. S2CID 125849923.
- ^ Beiser 2003, p. 432–433.
- ^ a b c Oganessian, Yu. (2012). "Nuclei in the "Island of Stability" of Superheavy Elements". Journal of Physics: Conference Series. 337 (1): 012005-1–012005-6. Bibcode:2012JPhCS.337a2005O. doi:10.1088/1742-6596/337/1/012005. ISSN 1742-6596.
- ^ Moller, P.; Nix, J. R. (1994). Fission properties of the heaviest elements (PDF). Dai 2 Kai Hadoron Tataikei no Simulation Symposium, Tokai-mura, Ibaraki, Japan. University of North Texas. Retrieved 16 February 2020.
- ^ a b Oganessian, Yu. Ts. (2004). "Superheavy elements". Physics World. 17 (7): 25–29. doi:10.1088/2058-7058/17/7/31. Retrieved 16 February 2020.
- ^ Schädel, M. (2015). "Chemistry of the superheavy elements". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 373 (2037): 20140191. Bibcode:2015RSPTA.37340191S. doi:10.1098/rsta.2014.0191. ISSN 1364-503X. PMID 25666065.
- ^ Hulet, E. K. (1989). Biomodal spontaneous fission. 50th Anniversary of Nuclear Fission, Leningrad, USSR. Bibcode:1989nufi.rept...16H.
- ^ Oganessian, Yu. Ts.; Rykaczewski, K. P. (2015). "A beachhead on the island of stability". Physics Today. 68 (8): 32–38. Bibcode:2015PhT....68h..32O. doi:10.1063/PT.3.2880. ISSN 0031-9228. OSTI 1337838. S2CID 119531411.
- ^ Grant, A. (2018). "Weighing the heaviest elements". Physics Today. doi:10.1063/PT.6.1.20181113a. S2CID 239775403.
- ^ Howes, L. (2019). "Exploring the superheavy elements at the end of the periodic table". Chemical & Engineering News. Retrieved 27 January 2020.
- ^ a b Robinson, A. E. (2019). "The Transfermium Wars: Scientific Brawling and Name-Calling during the Cold War". Distillations. Retrieved 22 February 2020.
- ^ "Популярная библиотека химических элементов. Сиборгий (экавольфрам)" [Popular library of chemical elements. Seaborgium (eka-tungsten)]. n-t.ru (in Russian). Retrieved 7 January 2020. Reprinted from "Экавольфрам" [Eka-tungsten]. Популярная библиотека химических элементов. Серебро – Нильсборий и далее [Popular library of chemical elements. Silver through nielsbohrium and beyond] (in Russian). Nauka. 1977.
- ^ "Nobelium - Element information, properties and uses | Periodic Table". Royal Society of Chemistry. Retrieved 1 March 2020.
- ^ a b Kragh 2018, pp. 38–39.
- ^ Kragh 2018, p. 40.
- ^ a b Ghiorso, A.; Seaborg, G. T.; Oganessian, Yu. Ts.; et al. (1993). "Responses on the report 'Discovery of the Transfermium elements' followed by reply to the responses by Transfermium Working Group" (PDF). Pure and Applied Chemistry. 65 (8): 1815–1824. doi:10.1351/pac199365081815. S2CID 95069384. Archived (PDF) from the original on 25 November 2013. Retrieved 7 September 2016.
- ^ Commission on Nomenclature of Inorganic Chemistry (1997). "Names and symbols of transfermium elements (IUPAC Recommendations 1997)" (PDF). Pure and Applied Chemistry. 69 (12): 2471–2474. doi:10.1351/pac199769122471.
- ^ Fleischmann, Martin; Pons, Stanley (1989). "Electrochemically induced nuclear fusion of deuterium". Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 261 (2): 301–308. doi:10.1016/0022-0728(89)80006-3.
- ^ Barber, Robert C.; Gäggeler, Heinz W.; Karol, Paul J.; Nakahara, Hiromichi; Vardaci, Emanuele; Vogt, Erich (2009). "Discovery of the element with atomic number 112 (IUPAC Technical Report)". Pure and Applied Chemistry. 81 (7): 1331. doi:10.1351/PAC-REP-08-03-05.
- ^ Armbruster, Peter; Munzenberg, Gottfried (1989). "Creating superheavy elements". Scientific American. 34: 36–42.
- ^ a b c d e f g Chapman, Kit (30 November 2016). "What it takes to make a new element". Chemistry World. Royal Society of Chemistry. Retrieved 3 December 2016.
- ^ Hofmann, Sigurd (2016). The discovery of elements 107 to 112 (PDF). Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements. doi:10.1051/epjconf/201613106001.
- ^ Oganessian, Yu. Ts.; et al. (1999). "Synthesis of Superheavy Nuclei in the 48Ca + 244Pu Reaction" (PDF). Physical Review Letters. 83 (16): 3154. Bibcode:1999PhRvL..83.3154O. doi:10.1103/PhysRevLett.83.3154. Archived from the original (PDF) on 30 July 2020. Retrieved 5 April 2017.
- ^ Oganessian, Yu. Ts.; et al. (2004). "Measurements of cross sections and decay properties of the isotopes of elements 112, 114, and 116 produced in the fusion reactions 233,238U, 242Pu, and 248Cm + 48Ca" (PDF). Physical Review C. 70 (6): 064609. Bibcode:2004PhRvC..70f4609O. doi:10.1103/PhysRevC.70.064609. Archived from the original (PDF) on 28 May 2008.
- ^ Oganessian, Yu. Ts.; et al. (2000). "Synthesis of superheavy nuclei in the 48Ca + 244Pu reaction: 288114". Physical Review C. 62 (4): 041604. Bibcode:2000PhRvC..62d1604O. doi:10.1103/PhysRevC.62.041604.
- ^ Oganessian, Yu. Ts.; et al. (2004). "Measurements of cross sections for the fusion-evaporation reactions 244Pu(48Ca,xn)292−x114 and 245Cm(48Ca,xn)293−x116". Physical Review C. 69 (5): 054607. Bibcode:2004PhRvC..69e4607O. doi:10.1103/PhysRevC.69.054607.
- ^ a b c Oganessian, Yu. Ts.; Utyonkoy, V.; Lobanov, Yu.; Abdullin, F.; Polyakov, A.; Shirokovsky, I.; Tsyganov, Yu.; Gulbekian, G.; Bogomolov, S.; Mezentsev, A. N.; et al. (2004). "Experiments on the synthesis of element 115 in the reaction 243Am(48Ca,xn)291−x115" (PDF). Physical Review C. 69 (2): 021601. Bibcode:2004PhRvC..69b1601O. doi:10.1103/PhysRevC.69.021601. Archived from the original (PDF) on 7 March 2020. Retrieved 13 December 2019.
- ^ Morita, Kōsuke (5 February 2016). "Q & A session". The Foreign Correspondents' Club of Japan. Archived from the original on 14 November 2021. Retrieved 28 April 2017 – via YouTube.
- ^ a b c d e f g Barber, Robert C.; Karol, Paul J; Nakahara, Hiromichi; Vardaci, Emanuele; Vogt, Erich W. (2011). "Discovery of the elements with atomic numbers greater than or equal to 113 (IUPAC Technical Report)". Pure Appl. Chem. 83 (7): 1485. doi:10.1351/PAC-REP-10-05-01.
- ^ a b Rudolph, D.; Forsberg, U.; Golubev, P.; Sarmiento, L. G.; Yakushev, A.; Andersson, L.-L.; Di Nitto, A.; Düllmann, Ch. E.; Gates, J. M.; Gregorich, K. E.; Gross, C. J.; Heßberger, F. P.; Herzberg, R.-D.; Khuyagbaatar, J.; Kratz, J. V.; Rykaczewski, K.; Schädel, M.; Åberg, S.; Ackermann, D.; Block, M.; Brand, H.; Carlsson, B. G.; Cox, D.; Derkx, X.; Eberhardt, K.; Even, J.; Fahlander, C.; Gerl, J.; Jäger, E.; Kindler, B.; Krier, J.; Kojouharov, I.; Kurz, N.; Lommel, B.; Mistry, A.; Mokry, C.; Nitsche, H.; Omtvedt, J. P.; Papadakis, P.; Ragnarsson, I.; Runke, J.; Schaffner, H.; Schausten, B.; Thörle-Pospiech, P.; Torres, T.; Traut, T.; Trautmann, N.; Türler, A.; Ward, A.; Ward, D. E.; Wiehl, N. (2013). "Spectroscopy of Element 115 Decay Chains". Physical Review Letters (Submitted manuscript). 111 (11): 112502. Bibcode:2013PhRvL.111k2502R. doi:10.1103/PhysRevLett.111.112502. ISSN 0031-9007. PMID 24074079. S2CID 3838065.
- ^ a b Morita, Kosuke; Morimoto, Kouji; Kaji, Daiya; Akiyama, Takahiro; Goto, Sin-ichi; Haba, Hiromitsu; Ideguchi, Eiji; Kanungo, Rituparna; Katori, Kenji; Koura, Hiroyuki; Kudo, Hisaaki; Ohnishi, Tetsuya; Ozawa, Akira; Suda, Toshimi; Sueki, Keisuke; Xu, HuShan; Yamaguchi, Takayuki; Yoneda, Akira; Yoshida, Atsushi; Zhao, YuLiang (2004). "Experiment on the Synthesis of Element 113 in the Reaction 209Bi(70Zn,n)278113". Journal of the Physical Society of Japan. 73 (10): 2593–2596. Bibcode:2004JPSJ...73.2593M. doi:10.1143/JPSJ.73.2593.
- ^ a b c d e f Karol, Paul J.; Barber, Robert C.; Sherrill, Bradley M.; Vardaci, Emanuele; Yamazaki, Toshimitsu (22 December 2015). "Discovery of the elements with atomic numbers Z = 113, 115 and 117 (IUPAC Technical Report)". Pure Appl. Chem. 88 (1–2): 139–153. doi:10.1515/pac-2015-0502.
- ^ a b Dmitriev, S. N.; Oganessyan, Yu. Ts.; Utyonkov, V. K.; Shishkin, S. V.; Yeremin, A. V.; Lobanov, Yu. V.; Tsyganov, Yu. S.; Chepygin, V. I.; Sokol, E. A.; Vostokin, G. K.; Aksenov, N. V.; Hussonnois, M.; Itkis, M. G.; Gäggeler, H. W.; Schumann, D.; Bruchertseifer, H.; Eichler, R.; Shaughnessy, D. A.; Wilk, P. A.; Kenneally, J. M.; Stoyer, M. A.; Wild, J. F. (2005). "Chemical identification of dubnium as a decay product of element 115 produced in the reaction 48Ca+243Am". Mendeleev Communications. 15 (1): 1–4. doi:10.1070/MC2005v015n01ABEH002077. S2CID 98386272.
- ^ Oganessian, Yu. Ts.; Utyonkov, V.; Dmitriev, S.; Lobanov, Yu.; Itkis, M.; Polyakov, A.; Tsyganov, Yu.; Mezentsev, A.; Yeremin, A.; Voinov, A. A.; et al. (2005). "Synthesis of elements 115 and 113 in the reaction 243Am + 48Ca". Physical Review C. 72 (3): 034611. Bibcode:2005PhRvC..72c4611O. doi:10.1103/PhysRevC.72.034611.
- ^ a b c Morimoto, Kouji (2016). "The discovery of element 113 at RIKEN" (PDF). 26th International Nuclear Physics Conference. Retrieved 14 May 2017.
- ^ Oganessian, Yu. Ts.; Utyonkov, V.; Lobanov, Yu.; Abdullin, F.; Polyakov, A.; Sagaidak, R.; Shirokovsky, I.; Tsyganov, Yu.; Voinov, A.; Gulbekian, Gulbekian; et al. (2007). "Synthesis of the isotope 282113 in the 237Np + 48Ca fusion reaction" (PDF). Physical Review C. 76 (1): 011601(R). Bibcode:2007PhRvC..76a1601O. doi:10.1103/PhysRevC.76.011601.
- ^ Morita, Kosuke; Morimoto, Kouji; Kaji, Daiya; Haba, Hiromitsu; Ozeki, Kazutaka; Kudou, Yuki; Sato, Nozomi; Sumita, Takayuki; Yoneda, Akira; Ichikawa, Takatoshi; Fujimori, Yasuyuki; Goto, Sin-ichi; Ideguchi, Eiji; Kasamatsu, Yoshitaka; Katori, Kenji; Komori, Yukiko; Koura, Hiroyuki; Kudo, Hisaaki; Ooe, Kazuhiro; Ozawa, Akira; Tokanai, Fuyuki; Tsukada, Kazuaki; Yamaguchi, Takayuki; Yoshida, Atsushi (25 May 2009). "Decay Properties of 266Bh and 262Db Produced in the 248Cm + 23Na Reaction". Journal of the Physical Society of Japan. 78 (6): 064201–1–6. arXiv:0904.1093. Bibcode:2009JPSJ...78f4201M. doi:10.1143/JPSJ.78.064201. S2CID 16415500.
- ^ Morimoto, Kouji; Morita, K.; Kaji, D.; Haba, H.; Ozeki, K.; Kudou, Y.; Sato, N.; Sumita, T.; Yoneda, A.; Ichikawa, T.; Fujimori, Y.; Goto, S.; Ideguchi, E.; Kasamatsu, Y.; Katori, K.; Komori, Y.; Koura, H.; Kudo, H.; Ooe, K.; Ozawa, A.; Tokanai, F.; Tsukada, K.; Yamaguchi, T.; Yoshida, A. (October 2009). "Production and Decay Properties of 266Bh and its daughter nuclei by using the 248Cm(23Na,5n)266Bh Reaction" (PDF). Archived from the original (PDF) on 21 September 2017. Retrieved 28 April 2017 – via University of Mainz.
- ^ Oganessian, Yuri Ts.; Abdullin, F. Sh.; Bailey, P. D.; Benker, D. E.; Bennett, M. E.; Dmitriev, S. N.; Ezold, J. G.; Hamilton, J. H.; Henderson, R. A.; Itkis, M. G.; Lobanov, Yuri V.; Mezentsev, A. N.; Moody, K. J.; Nelson, S. L.; Polyakov, A. N.; Porter, C. E.; Ramayya, A. V.; Riley, F. D.; Roberto, J. B.; Ryabinin, M. A.; Rykaczewski, K. P.; Sagaidak, R. N.; Shaughnessy, D. A.; Shirokovsky, I. V.; Stoyer, M. A.; Subbotin, V. G.; Sudowe, R.; Sukhov, A. M.; Tsyganov, Yu. S.; Utyonkov, Vladimir K.; Voinov, A. A.; Vostokin, G. K.; Wilk, P. A. (9 April 2010). "Synthesis of a New Element with Atomic Number Z=117". Physical Review Letters. 104 (14): 142502. Bibcode:2010PhRvL.104n2502O. doi:10.1103/PhysRevLett.104.142502. PMID 20481935.
- ^ a b K. Morita; Morimoto, Kouji; Kaji, Daiya; Haba, Hiromitsu; Ozeki, Kazutaka; Kudou, Yuki; Sumita, Takayuki; Wakabayashi, Yasuo; Yoneda, Akira; Tanaka, Kengo; et al. (2012). "New Results in the Production and Decay of an Isotope, 278113, of the 113th Element". Journal of the Physical Society of Japan. 81 (10): 103201. arXiv:1209.6431. Bibcode:2012JPSJ...81j3201M. doi:10.1143/JPSJ.81.103201. S2CID 119217928.
- ^ a b Chapman, Kit (8 February 2018). "Nihonium". Chemistry World. Royal Society of Chemistry. Retrieved 20 March 2018.
- ^ Morita, Kosuke (2015). "SHE Research at RIKEN/GARIS" (PDF). Retrieved 4 September 2018 – via Texas A&M University Cyclotron Institute.
- ^ "Existence of new element confirmed". Lund University. 27 August 2013. Retrieved 10 April 2016.
- ^ Gates, J. M.; Gregorich, K. E.; Gothe, O. .R; Uribe, E. C.; Pang, G. K.; Bleuel, D. L.; Block, M.; Clark, R. M.; Campbell, C. M.; Crawford, H. L.; Cromaz, M.; Di Nitto, A.; Düllmann, Ch. E.; Esker, N. E.; Fahlander, C.; Fallon, P.; Farjadi, R. M.; Forsberg, U.; Khuyagbaatar, J.; Loveland, W.; MacChiavelli, A. O.; May, E. M.; Mudder, P. R.; Olive, D. T.; Rice, A. C.; Rissanen, J.; Rudolph, D.; Sarmiento, L. G.; Shusterman, J. A.; et al. (2015). "Decay spectroscopy of element 115 daughters: 280Rg→276Mt and 276Mt→Bh". Physical Review C. 92 (2): 021301. Bibcode:2015PhRvC..92b1301G. doi:10.1103/PhysRevC.92.021301.
- ^ a b "Element 113: Ununtrium Reportedly Synthesised In Japan". Huffington Post. September 2012. Retrieved 22 April 2013.
- ^ a b McKellar, Bruce (22–23 October 2016). "President's report to the meeting of the IUPAP Council and Commission Chairs" (PDF). International Union of Pure and Applied Physics. Archived from the original (PDF) on 2 November 2020. Retrieved 14 January 2018.
- ^ a b "Discovery of the new chemical elements with numbers 113, 115, 117 and 118". Joint Institute for Nuclear Research. 6 January 2016. Retrieved 14 January 2018.
- ^ a b c "Discovery and Assignment of Elements with Atomic Numbers 113, 115, 117 and 118". IUPAC. 30 December 2015. Archived from the original on 31 December 2015. Retrieved 8 September 2018.
- ^ Forsberg, U.; Rudolph, D.; Fahlander, C.; Golubev, P.; Sarmiento, L. G.; Åberg, S.; Block, M.; Düllmann, Ch. E.; Heßberger, F. P.; Kratz, J. V.; Yakushev, A. (9 July 2016). "A new assessment of the alleged link between element 115 and element 117 decay chains" (PDF). Physics Letters B. 760 (2016): 293–296. Bibcode:2016PhLB..760..293F. doi:10.1016/j.physletb.2016.07.008. Retrieved 2 April 2016.
- ^ Forsberg, Ulrika; Fahlander, Claes; Rudolph, Dirk (2016). Congruence of decay chains of elements 113, 115, and 117 (PDF). Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements. doi:10.1051/epjconf/201613102003.
- ^ Zlokazov, V. B.; Utyonkov, V. K. (8 June 2017). "Analysis of decay chains of superheavy nuclei produced in the 249Bk + 48Ca and 243Am + 48Ca reactions". Journal of Physics G: Nuclear and Particle Physics. 44 (75107): 075107. Bibcode:2017JPhG...44g5107Z. doi:10.1088/1361-6471/aa7293.
- ^ Chatt, J. (1979). "Recommendations for the Naming of Elements of Atomic Numbers Greater than 100". Pure Appl. Chem. 51 (2): 381–384. doi:10.1351/pac197951020381.
- ^ a b Noorden, Richard Van (27 September 2012). "Element 113 at Last?". Scientific American.
- ^ 新元素113番、日本の発見確実に 合成に3回成功. Nihon Keizai Shimbun (in Japanese). 27 September 2012. Retrieved 13 October 2012.
- ^ "Proposed name for 113th element a fulfilled wish for Japanese researchers". The Mainichi. 9 June 2016. Retrieved 29 April 2018.
- ^ "Naming 113th element 'nihonium' a tribute to Japanese public support: researcher". The Mainichi. 9 June 2016. Retrieved 29 April 2018.
- ^ a b "IUPAC Is Naming The Four New Elements Nihonium, Moscovium, Tennessine, And Oganesson". IUPAC. 8 June 2016. Retrieved 8 June 2016.
- ^ Ikeda, Nagao (25 July 2011). "The discoveries of uranium 237 and symmetric fission – From the archival papers of Nishina and Kimura". Proceedings of the Japan Academy, Series B: Physical and Biological Sciences. 87 (7): 371–376. Bibcode:2011PJAB...87..371I. doi:10.2183/pjab.87.371. PMC 3171289. PMID 21785255.
- ^ En'yo, Hideto (26 May 2017). "Bikkuban kara 113-ban genso nihoniumu made, genso sōsei no 138 oku-nen" ビックバンから 113番元素ニホニウムまで、元素創成の138億年 [From the Big Bang to the 113th element nihonium: element creation of 13.8 billion years] (PDF) (in Japanese). Archived from the original (PDF) on 29 January 2018. Retrieved 28 January 2018.
- ^ "Japan scientists plan to name atomic element 113 'Nihonium'". Mainichi Shimbun. 8 June 2016. Archived from the original on 9 June 2016.
Japanese scientists who discovered the atomic element 113 plan to name it "Nihonium", sources close to the matter said Wednesday.
- ^ "ニホニウム」有力 日本初の新元素名称案、国際機関が9日公表" [Nihonium the most probable]. The Sankei Shimbun (in Japanese). 6 June 2016.
Rather than initially proposed Japanium which is derived from Latin or French, Morita group leader seems to stick to his own language.
- ^ "IUPAC Announces the Names of the Elements 113, 115, 117, and 118". IUPAC. 30 November 2016. Retrieved 30 November 2016.
- ^ "Naming ceremony held for new element 'nihonium'". News on Japan. 15 March 2017. Archived from the original on 28 January 2018. Retrieved 28 January 2018.
- ^ a b c d e Oganessian, Yu. Ts.; Utyonkov, V. K.; Kovrizhnykh, N. D.; et al. (2022). "New isotope 286Mc produced in the 243Am+48Ca reaction". Physical Review C. 106 (64306): 064306. Bibcode:2022PhRvC.106f4306O. doi:10.1103/PhysRevC.106.064306. S2CID 254435744.
- ^ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. Archived from the original on 7 August 2007. Retrieved 6 June 2008.
- ^ Forsberg, Ulrika (September 2016). "Recoil-α-fission and recoil-α–α-fission events observed in the reaction 48Ca + 243Am". Nuclear Physics A. 953: 117–138. arXiv:1502.03030. Bibcode:2016NuPhA.953..117F. doi:10.1016/j.nuclphysa.2016.04.025. S2CID 55598355.
- ^ Considine, Douglas M.; Considine, Glenn D. (1994). Van Nostrand's Scientific Encyclopedia (8th ed.). Wiley-Interscience. p. 623. ISBN 978-1-4757-6918-0.
- ^ a b Oganessian, Yu. Ts.; Sobiczewski, A.; Ter-Akopian, G. M. (9 January 2017). "Superheavy nuclei: from predictions to discovery". Physica Scripta. 92 (2): 023003–1–21. Bibcode:2017PhyS...92b3003O. doi:10.1088/1402-4896/aa53c1. S2CID 125713877.
- ^ Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ a b Zagrebaev, Karpov & Greiner 2013, pp. 1–15.
- ^ Ibadullayev, Dastan (2024). "Synthesis and study of the decay properties of isotopes of superheavy element Lv in Reactions 238U + 54Cr and 242Pu + 50Ti". jinr.ru. Joint Institute for Nuclear Research. Retrieved 2 November 2024.
- ^ Hong, J.; Adamian, G. G.; Antonenko, N. V.; Jachimowicz, P.; Kowal, M. (26 April 2023). Interesting fusion reactions in superheavy region (PDF). IUPAP Conference "Heaviest nuclei and atoms". Joint Institute for Nuclear Research. Retrieved 30 July 2023.
- ^ Hong, J.; Adamian, G. G.; Antonenko, N. V. (2017). "Ways to produce new superheavy isotopes with Z = 111–117 in charged particle evaporation channels". Physics Letters B. 764: 42–48. Bibcode:2017PhLB..764...42H. doi:10.1016/j.physletb.2016.11.002.
- ^ Chapman, Kit (30 November 2016). "What it takes to make a new element". Chemistry World. Retrieved 26 June 2024.
- ^ a b c d Stysziński, Jacek (2010). "Why do we Need Relativistic Computational Methods?". Relativistic Methods for Chemists. Challenges and Advances in Computational Chemistry and Physics. Vol. 10. pp. 139–146. doi:10.1007/978-1-4020-9975-5_3. ISBN 978-1-4020-9974-8.
- ^ a b c Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements". Relativistic Methods for Chemists. Challenges and Advances in Computational Chemistry and Physics. Vol. 10. pp. 63–97. doi:10.1007/978-1-4020-9975-5_2. ISBN 978-1-4020-9974-8.
- ^ a b Fægri Jr., Knut; Saue, Trond (2001). "Diatomic molecules between very heavy elements of group 13 and group 17: A study of relativistic effects on bonding". The Journal of Chemical Physics. 115 (6): 2456. Bibcode:2001JChPh.115.2456F. doi:10.1063/1.1385366.
- ^ a b Zaitsevskii, A.; van Wüllen, C.; Rusakov, A.; Titov, A. (September 2007). "Relativistic DFT and ab initio calculations on the seventh-row superheavy elements: E113 – E114" (PDF). Retrieved 17 February 2018.
- ^ Han, Young-Kyu; Bae, Cheolbeom; Son, Sang-Kil; Lee, Yoon Sup (2000). "Spin–orbit effects on the transactinide p-block element monohydrides MH (M=element 113–118)". Journal of Chemical Physics. 112 (6): 2684. Bibcode:2000JChPh.112.2684H. doi:10.1063/1.480842. S2CID 9959620.
- ^ a b c d e f Seth, Michael; Schwerdtfeger, Peter; Fægri, Knut (1999). "The chemistry of superheavy elements. III. Theoretical studies on element 113 compounds". Journal of Chemical Physics. 111 (14): 6422–6433. Bibcode:1999JChPh.111.6422S. doi:10.1063/1.480168. hdl:2292/5178. S2CID 41854842.
- ^ Demidov, Yu. A. (15 February 2017). "Quantum chemical modelling of electronic structure of nihonium and astatine compounds". Flerov Laboratory of Nuclear Reactions. Retrieved 12 June 2017.
- ^ Nash, Clinton S.; Bursten, Bruce E. (1999). "Spin−Orbit Effects, VSEPR Theory, and the Electronic Structures of Heavy and Superheavy Group IVA Hydrides and Group VIIIA Tetrafluorides. A Partial Role Reversal for Elements 114 and 118". J. Phys. Chem. A. 103 (3): 402–410. Bibcode:1999JPCA..103..402N. doi:10.1021/jp982735k. PMID 27676357.
- ^ a b c d e Eichler, Robert (2013). "First foot prints of chemistry on the shore of the Island of Superheavy Elements". Journal of Physics: Conference Series. 420 (1): 012003. arXiv:1212.4292. Bibcode:2013JPhCS.420a2003E. doi:10.1088/1742-6596/420/1/012003. S2CID 55653705.
- ^ Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 195, 233–235, 237–240. ISBN 978-0-7506-3365-9.
- ^ Downs, A.J. (31 May 1993). Chemistry of Aluminium, Gallium, Indium and Thallium. Springer Science & Business Media. pp. 128–137. ISBN 978-0-7514-0103-5.
- ^ Bae, Ch.; Han, Y.-K.; Lee, Yo. S. (18 January 2003). "Spin−Orbit and Relativistic Effects on Structures and Stabilities of Group 17 Fluorides EF3 (E = I, At, and Element 117): Relativity Induced Stability for the D3h Structure of (117)F3". The Journal of Physical Chemistry A. 107 (6): 852–858. Bibcode:2003JPCA..107..852B. doi:10.1021/jp026531m.
- ^ Tebbe, K.-F.; Georgy, U. (December 1986). "Die Kristallstrukturen von Rubidiumtriiodid und Thalliumtriiodid". Acta Crystallographica C. C42 (12): 1675–1678. Bibcode:1986AcCrC..42.1675T. doi:10.1107/S0108270186090972.
- ^ Düllmann, Christoph E. (2012). "Superheavy elements at GSI: a broad research program with element 114 in the focus of physics and chemistry". Radiochimica Acta. 100 (2): 67–74. doi:10.1524/ract.2011.1842. S2CID 100778491.
- ^ Moody, Ken (30 November 2013). "Synthesis of Superheavy Elements". In Schädel, Matthias; Shaughnessy, Dawn (eds.). The Chemistry of Superheavy Elements (2nd ed.). Springer Science & Business Media. pp. 24–28. ISBN 978-3-642-37466-1.
- ^ Tereshatov, E. E.; Boltoeva, M. Yu.; Folden III, C. M. (2015). "Resin Ion Exchange and Liquid-Liquid Extraction of Indium and Thallium from Chloride Media". Solvent Extraction and Ion Exchange. 33 (6): 607. doi:10.1080/07366299.2015.1080529. S2CID 94078206.
Bibliography
[edit]- Audi, G.; Kondev, F. G.; Wang, M.; et al. (2017). "The NUBASE2016 evaluation of nuclear properties". Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- Beiser, A. (2003). Concepts of modern physics (6th ed.). McGraw-Hill. ISBN 978-0-07-244848-1. OCLC 48965418.
- Hoffman, D. C.; Ghiorso, A.; Seaborg, G. T. (2000). The Transuranium People: The Inside Story. World Scientific. ISBN 978-1-78-326244-1.
- Kragh, H. (2018). From Transuranic to Superheavy Elements: A Story of Dispute and Creation. Springer. ISBN 978-3-319-75813-8.
- Zagrebaev, V.; Karpov, A.; Greiner, W. (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?". Journal of Physics: Conference Series. 420 (1): 012001. arXiv:1207.5700. Bibcode:2013JPhCS.420a2001Z. doi:10.1088/1742-6596/420/1/012001. ISSN 1742-6588. S2CID 55434734.
External links
[edit]- Nihonium at The Periodic Table of Videos (University of Nottingham)
- Uut and Uup Add Their Atomic Mass to Periodic Table Archived 7 September 2006 at the Wayback Machine
- Discovery of Elements 113 and 115
- Superheavy elements
- WebElements.com: Nihonium


