2-Methyltetrahydrofuran

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyloxolane | |
| Other names
2-Methyltetrahydrofuran, 2-Methyl-THF
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.281 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2536 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C5H10O | |
| Molar mass | 86.134 g·mol−1 |
| Density | 0.854 g/mL |
| Melting point | −136 °C (−213 °F; 137 K)[2] |
| Boiling point | 80.2 °C (176.4 °F; 353.3 K)[2] |
| 21.0 wt% (0.0 °C) 17.8 wt% (9.5 °C) 14.4 wt% (19.3 °C) 11.4 wt% (29.5 °C) 9.2 wt% (39.6 °C) 7.8 wt% (50.1 °C) 6.6 wt%(60.7 °C) 6.0 wt% (70.6 °C)[1] | |
| Hazards | |
| GHS labelling: | |
  
| |
| Warning | |
| H225, H302, H315, H318, H319, H335, H336 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+P312, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P330, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Methyltetrahydrofuran (2-MeTHF) is an organic compound with the molecular formula C5H10O. It is a highly flammable, mobile liquid. It is mainly used as a replacement for Tetrahydrofuran (THF) in specialized applications for its better performance, such as to obtain higher reaction temperatures, or easier separations (as, unlike THF, it is not miscible with water). It is derived from sugars via furfural and is occasionally touted as a biofuel.[3]
Structures and properties
[edit]2-Methyltetrahydrofuran is "inversely soluble" in water. That is, its solubility decreases with increasing temperature, which is a rare property.[4] The solubility of water in 2-methyltetrahydrofuran is reported to be 4.4 g/ 100 g at 23 °C.[5] Much like tetrahydrofuran, 2-methyltetrahydrofuran can act as a Lewis base in organometallic reactions.[2] 2-Methyltetrahydrofuran is usually produced as a racemic mixture.
Preparation
[edit]2-Methyltetrahydrofuran is usually synthesized by catalytic hydrogenation of furfural.[6]
- OC4H3CHO + 4 H2 → OC4H7CH3 + H2O
Furfural is produced by the acid-catalyzed digestion of pentosan sugars, C5 polysaccharides, in biomass. Thus, the raw materials of 2-methyltetrahydrofuran are renewable biomass rich with cellulose, hemicelluloses, and lignin, such as corncobs or bagasse and other plant and agricultural waste.[7]
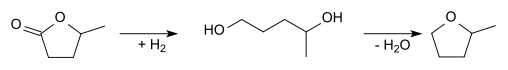
2-Methyltetrahydrofuran can also be produced starting from levulinic acid. Cyclization and reduction gives γ-valerolactone:
γ-Valerolactone can be hydrogenated to 1,4-pentanediol, which can then be dehydrated to give 2-methyltetrahydrofuran:
Applications
[edit]2-Methyltetrahydrofuran is mainly used as a higher boiling substitute for tetrahydrofuran as a specialty solvent. It also is used in the electrolyte formulation for secondary lithium electrodes and as a component in alternative fuels. It is a valued solvent for low-temperature reactions. 2-Methyltetrahydrofuran forms a glass, which does not crystallize, and is frequently used as a solvent for spectroscopic studies at −196 °C.[2]
Other common uses of 2-methyltetrahydrofuran is as a solvent for Grignard reagents used in organometallic and biphasic chemical processes, because of the oxygen atom's ability to coordinate to the magnesium ion component of the Grignard reagent, or to azeotropically dry products. The use of 2-methyltetrahydrofuran provides very clean organic water phase separations. It is a popular, but costlier substitute for tetrahydrofuran.
2-Methyltetrahydrofuran is approved by the United States Department of Energy as an additive to gasoline. Furfural and other furyl compounds (furfuryl alcohol, methylfuran, tetrahydrofufuryl alcohol) have a tendency to polymerize and are quite volatile. 2-Methyltetrahydrofuran itself, however, is more stable and less volatile, and thus is suitable for use as a motor fuel.
2-Methyltetrahydrofuran has been promoted as an ecologically-friendly alternative to THF.[8] Whereas 2-MeTHF is more expensive, it may provide for greater overall process economy. 2-MeTHF has solvating properties that are intermediate between diethyl ether and THF, has limited water miscibility, and forms an azeotrope with water on distillation. Its lower melting point makes it useful for lower temperature reactions, and its higher boiling point allows procedures under reflux at higher temperatures (relative to THF).
References
[edit]- ^ Sicaire, Anne-Gaëlle; Vian, Maryline Abert; Filly, Aurore; Li, Ying; Bily, Antoine; Chemat, Farid (2014), Chemat, Farid; Vian, Maryline Abert (eds.), "2-Methyltetrahydrofuran: Main Properties, Production Processes, and Application in Extraction of Natural Products", Alternative Solvents for Natural Products Extraction, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 253–268, doi:10.1007/978-3-662-43628-8_12, ISBN 978-3-662-43627-1
- ^ a b c d Aycock, David F. (2007). "Solvent Applications of 2-Methyltetrahydrofuran in Organometallic and Biphasic Reactions". Org. Process Res. Dev. 11: 156–159. doi:10.1021/op060155c.
- ^ Leal Silva, Jean Felipe; Mariano, Adriano Pinto; Maciel Filho, Rubens (2018). "Economic potential of 2-methyltetrahydrofuran (MTHF) and ethyl levulinate (EL) produced from hemicelluloses-derived furfural". Biomass and Bioenergy. 119 (December 2018): 492–502. Bibcode:2018BmBe..119..492L. doi:10.1016/j.biombioe.2018.10.008. S2CID 104836889.
- ^ Sicaire, Anne-Gaëlle; Vian, Maryline Abert; Filly, Aurore; Li, Ying; Bily, Antoine; Chemat, Farid (2014), Chemat, Farid; Vian, Maryline Abert (eds.), "2-Methyltetrahydrofuran: Main Properties, Production Processes, and Application in Extraction of Natural Products", Alternative Solvents for Natural Products Extraction, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 253–268, doi:10.1007/978-3-662-43628-8_12, ISBN 978-3-662-43627-1
- ^ "Zeon Corporation" (PDF). Archived from the original (PDF) on 2011-07-22. Retrieved 2024-08-05.
- ^ Huber, GW; Iborra, S; Corma, A (September 2006). "Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering". Chem. Rev. 106 (9): 4044–98. doi:10.1021/cr068360d. PMID 16967928. ref 306
- ^ Hoydonckx, H. E.; Van Rhijn, W. M.; Van Rhijn, W.; De Vos, D. E.; Jacobs, P. A. "Furfural and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_119.pub2. ISBN 978-3527306732.
- ^ "Greener Solvent Alternatives – Brochure" (PDF). Sigmaaldrich.com. Retrieved 2010-02-15.
- Huber, George W.; Iborra, Sara; Corma, Avelino (2006). "Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering". Chemical Reviews. 106 (9): 4044–4098. doi:10.1021/cr068360d. PMID 16967928.
Further reading
[edit]- Zheng, Hong-Yan; Zhu, Yu-Lei; Teng, Bo-Tao; Bai, Zong-Qing; Zhang, Cheng-Hua; Xiang, Hong-Wei; Li, Yong-Wang (2006). "Towards understanding the reaction pathway in vapour phase hydrogenation of furfural to 2-methylfuran". Journal of Molecular Catalysis A: Chemical. 246 (1–2): 18–23. doi:10.1016/j.molcata.2005.10.003.
- https://cms.chempoint.com/ChemPoint/media/ChemPointSiteMedia/Emails/MeTHF-System-Advantages-in-Organometallic-Chemistry-and-Biphasic-Reactions.pdf