Melanoma
| Melanoma | |
|---|---|
| Other names | Malignant melanoma |
 | |
| Pronunciation | |
| Specialty | Oncology and dermatology |
| Symptoms | Mole that is increasing in size, has irregular edges, change in color, itchiness, or skin breakdown.[1] |
| Causes | Ultraviolet light (Sun, tanning devices)[2] |
| Risk factors | Family history, many moles, poor immune function[1] |
| Diagnostic method | Tissue biopsy[1] |
| Differential diagnosis | Seborrheic keratosis, lentigo, blue nevus, dermatofibroma[3] |
| Prevention | Sunscreen, avoiding UV light[2] |
| Treatment | Surgery[1] |
| Prognosis | Five-year survival rates in US 99% (localized), 25% (disseminated)[4] |
| Frequency | 3.1 million (2015)[5] |
| Deaths | 59,800 (2015)[6] |
Melanoma is the most dangerous type of skin cancer; it develops from the melanin-producing cells known as melanocytes.[1] It typically occurs in the skin, but may rarely occur in the mouth, intestines, or eye (uveal melanoma).[1][2]
In women, melanomas most commonly occur on the legs; while in men, on the back.[2] Melanoma is frequently referred to as malignant melanoma. However, the medical community stresses that there is no such thing as a 'benign melanoma' and recommends that the term 'malignant melanoma' should be avoided as redundant.[7][8][9]
About 25% of melanomas develop from moles.[2] Changes in a mole that can indicate melanoma include increase—especially rapid increase—in size, irregular edges, change in color, itchiness, or skin breakdown.[1]
The primary cause of melanoma is ultraviolet light (UV) exposure in those with low levels of the skin pigment melanin.[2][10] The UV light may be from the sun or other sources, such as tanning devices.[2] Those with many moles, a history of affected family members, and poor immune function are at greater risk.[1] A number of rare genetic conditions, such as xeroderma pigmentosum, also increase the risk.[11] Diagnosis is by biopsy and analysis of any skin lesion that has signs of being potentially cancerous.[1]
Avoiding UV light and using sunscreen in UV-bright sun conditions may prevent melanoma.[2] Treatment typically is removal by surgery of the melanoma and the potentially affected adjacent tissue bordering the melanoma.[1] In those with slightly larger cancers, nearby lymph nodes may be tested for spread (metastasis).[1] Most people are cured if metastasis has not occurred.[1] For those in whom melanoma has spread, immunotherapy, biologic therapy, radiation therapy, or chemotherapy may improve survival.[1][12] With treatment, the five-year survival rates in the United States are 99% among those with localized disease, 65% when the disease has spread to lymph nodes, and 25% among those with distant spread.[4] The likelihood that melanoma will reoccur or spread depends on its thickness, how fast the cells are dividing, and whether or not the overlying skin has broken down.[2]
Melanoma is the most dangerous type of skin cancer.[2] Globally, in 2012, it newly occurred in 232,000 people.[2] In 2015, 3.1 million people had active disease, which resulted in 59,800 deaths.[5][6] Australia and New Zealand have the highest rates of melanoma in the world.[2] High rates also occur in Northern Europe and North America, while it is less common in Asia, Africa, and Latin America.[2] In the United States, melanoma occurs about 1.6 times more often in men than women.[13] Melanoma has become more common since the 1960s in areas mostly populated by people of European descent.[2][11]
Signs and symptoms
[edit]Early signs of melanoma are changes to the shape or color of existing moles or, in the case of nodular melanoma, the appearance of a new lump anywhere on the skin. At later stages, the mole may itch, ulcerate, or bleed. Early signs of melanoma are summarized by the mnemonic "ABCDEEFG":[14][15]
- Asymmetry
- Borders (irregular with edges and corners)
- Colour (variegated)
- Diameter (greater than 6 mm (0.24 in), about the size of a pencil eraser)
- Evolving over time
This classification does not apply to nodular melanoma, which has its own classifications:[16]
- Elevated above the skin surface
- Firm to the touch
- Growing
Metastatic melanoma may cause nonspecific paraneoplastic symptoms, including loss of appetite, nausea, vomiting, and fatigue. Metastasis (spread) of early melanoma is possible, but relatively rare; less than a fifth of melanomas diagnosed early become metastatic. Brain metastases are particularly common in patients with metastatic melanoma.[17] It can also spread to the liver, bones, abdomen, or distant lymph nodes.[citation needed]
Cause
[edit]Melanomas are usually caused by DNA damage resulting from exposure to UV light from the sun. Genetics also play a role.[18][19] Melanoma can also occur in skin areas with little sun exposure (i.e. mouth, soles of feet, palms of hands, genital areas).[20] People with dysplastic nevus syndrome, also known as familial atypical multiple mole melanoma, are at increased risk for the development of melanoma.[21]
Having more than 50 moles indicates an increased risk of melanoma. A weakened immune system makes cancer development easier due to the body's weakened ability to fight cancer cells.[18]
UV radiation
[edit]UV radiation exposure from tanning beds increases the risk of melanoma.[22] The International Agency for Research on Cancer finds that tanning beds are "carcinogenic to humans" and that people who begin using tanning devices before the age of thirty years are 75% more likely to develop melanoma.[23]
Those who work in airplanes also appear to have an increased risk, believed to be due to greater exposure to UV.[24]
UVB light, emanating from the sun at wavelengths between 315 and 280 nm, is absorbed directly by DNA in skin cells, which results in a type of direct DNA damage called cyclobutane pyrimidine dimers. Thymine, cytosine, or cytosine-thymine dimers are formed by the joining of two adjacent pyrimidine bases within a strand of DNA. UVA light presents at wavelengths longer than UVB (between 400 and 315 nm); and it can also be absorbed directly by DNA in skin cells, but at lower efficiencies—about 1/100 to 1/1000 of UVB.[25]
Exposure to radiation (UVA and UVB) is a major contributor to developing melanoma.[26] Occasional extreme sun exposure that results in "sunburn" on areas of the human body is causally related to melanoma;[27] and such areas of only intermittent exposure apparently explains why melanoma is more common on the back in men and on the legs in women. The risk appears to be strongly influenced by socioeconomic conditions rather than indoor versus outdoor occupations; it is more common in professional and administrative workers than unskilled workers.[28][29] Other factors are mutations in (or total loss of) tumor suppressor genes. Using sunbeds with their deeply penetrating UVA rays has been linked to the development of skin cancers, including melanoma.[30]
Possible significant elements in determining risk include the intensity and duration of sun exposure, the age at which sun exposure occurs, and the degree of skin pigmentation. Melanoma rates tend to be highest in countries settled by migrants from Europe which have a large amount of direct, intense sunlight to which the skin of the settlers is not adapted, most notably Australia. Exposure during childhood is a more important risk factor than exposure in adulthood. This is seen in migration studies in Australia.[31]
Incurring multiple severe sunburns increases the likelihood that future sunburns develop into melanoma due to cumulative damage.[18] UV-high sunlight and tanning beds are the main sources of UV radiation that increase the risk for melanoma[32] and living close to the equator increases exposure to UV radiation.[18]
Genetics
[edit]A number of rare mutations, which often run in families, greatly increase melanoma susceptibility.[33] Several genes increase risks. Some rare genes have a relatively high risk of causing melanoma; some more common genes, such as a gene called MC1R that causes red hair, have a relatively lower elevated risk. Genetic testing can be used to search for the mutations.[citation needed]
One class of mutations affects the gene CDKN2A. An alternative reading frame mutation in this gene leads to the destabilization of p53, a transcription factor involved in apoptosis and in 50% of human cancers. Another mutation in the same gene results in a nonfunctional inhibitor of CDK4, a cyclin-dependent kinase that promotes cell division. Mutations that cause the skin condition xeroderma pigmentosum (XP) also increase melanoma susceptibility. Scattered throughout the genome, these mutations reduce a cell's ability to repair DNA. Both CDKN2A and XP mutations are highly penetrant (the chances of a carrier to express the phenotype is high).[citation needed]
Familial melanoma is genetically heterogeneous,[19] and loci for familial melanoma appear on the chromosome arms 1p, 9p and 12q. Multiple genetic events have been related to melanoma's pathogenesis (disease development).[34] The multiple tumor suppressor 1 (CDKN2A/MTS1) gene encodes p16INK4a – a low-molecular weight protein inhibitor of cyclin-dependent protein kinases (CDKs) – which has been localised to the p21 region of human chromosome 9.[35] FAMMM is typically characterized by having 50 or more combined moles in addition to a family history of melanoma.[20] It is transmitted autosomal dominantly and mostly associated with the CDKN2A mutations.[20] People who have CDKN2A mutation associated FAMMM have a 38 fold increased risk of pancreatic cancer.[36]
Other mutations confer lower risk, but are more common in the population. People with mutations in the MC1R gene are two to four times more likely to develop melanoma than those with two wild-type (typical unaffected type) copies. MC1R mutations are very common, and all red-haired people have a mutated copy.[citation needed] Mutation of the MDM2 SNP309 gene is associated with increased risks for younger women.[37]
Fair- and red-haired people, persons with multiple atypical nevi or dysplastic nevi and persons born with giant congenital melanocytic nevi are at increased risk.[38]
A family history of melanoma greatly increases a person's risk, because mutations in several genes have been found in melanoma-prone families.[39][18] People with a history of one melanoma are at increased risk of developing a second primary tumor.[40]
Fair skin is the result of having less melanin in the skin, which means less protection from UV radiation exists.[18]
Pathophysiology
[edit]

The earliest stage of melanoma starts when melanocytes begin out-of-control growth. Melanocytes are found between the outer layer of the skin (the epidermis) and the next layer (the dermis). This early stage of the disease is called the radial growth phase, when the tumor is less than 1 mm thick, and spreads at the level of the basal epidermis.[41] Because the cancer cells have not yet reached the blood vessels deeper in the skin, it is very unlikely that this early-stage melanoma will spread to other parts of the body. If the melanoma is detected at this stage, then it can usually be completely removed with surgery.[citation needed]
When the tumor cells start to move in a different direction – vertically up into the epidermis and into the papillary dermis – cell behaviour changes dramatically.[42]
The next step in the evolution is the invasive radial growth phase, in which individual cells start to acquire invasive potential. From this point on, melanoma is capable of spreading.[citation needed] The Breslow's depth of the lesion is usually less than 1 mm (0.04 in), while the Clark level is usually 2.
The vertical growth phase (VGP) following is invasive melanoma. The tumor becomes able to grow into the surrounding tissue and can spread around the body through blood or lymph vessels. The tumor thickness is usually more than 1 mm (0.04 in), and the tumor involves the deeper parts of the dermis.
The host elicits an immunological reaction against the tumor during the VGP,[43] which is judged by the presence and activity of the tumor infiltrating lymphocytes (TILs). These cells sometimes completely destroy the primary tumor; this is called regression, which is the latest stage of development. In certain cases, the primary tumor is completely destroyed and only the metastatic tumor is discovered. About 40% of human melanomas contain activating mutations affecting the structure of the B-Raf protein, resulting in constitutive signaling through the Raf to MAP kinase pathway.[44]
A cause common to most cancers is damage to DNA.[45] UVA light mainly causes thymine dimers.[46] UVA also produces reactive oxygen species and these inflict other DNA damage, primarily single-strand breaks, oxidized pyrimidines and the oxidized purine 8-oxoguanine (a mutagenic DNA change) at 1/10, 1/10, and 1/3rd the frequencies of UVA-induced thymine dimers, respectively.
If unrepaired, cyclobutane pyrimidine dimer (CPD) photoproducts can lead to mutations by inaccurate translesion synthesis during DNA replication or repair. The most frequent mutations due to inaccurate synthesis past CPDs are cytosine to thymine (C>T) or CC>TT transition mutations. These are commonly referred to as UV fingerprint mutations, as they are the most specific mutation caused by UV, being frequently found in sun-exposed skin, but rarely found in internal organs.[47] Errors in DNA repair of UV photoproducts, or inaccurate synthesis past these photoproducts, can also lead to deletions, insertions, and chromosomal translocations.
The entire genomes of 25 melanomas were sequenced.[48] On average, about 80,000 mutated bases (mostly C>T transitions) and about 100 structural rearrangements were found per melanoma genome. This is much higher than the roughly 70 mutations across generations (parent to child).[49][50] Among the 25 melanomas, about 6,000 protein-coding genes had missense, nonsense, or splice site mutations. The transcriptomes of over 100 melanomas has also been sequenced and analyzed. Almost 70% of all human protein-coding genes are expressed in melanoma. Most of these genes are also expressed in other normal and cancer tissues, with some 200 genes showing a more specific expression pattern in melanoma compared to other forms of cancer. Examples of melanoma specific genes are tyrosinase, MLANA, and PMEL.[51][52]
UV radiation causes damage to the DNA of cells, typically thymine dimerization, which when unrepaired can create mutations in the cell's genes. This strong mutagenic factor makes cutaneous melanoma the tumor type with the highest number of mutations.[53] When the cell divides, these mutations are propagated to new generations of cells. If the mutations occur in protooncogenes or tumor suppressor genes, the rate of mitosis in the mutation-bearing cells can become uncontrolled, leading to the formation of a tumor. Data from patients suggest that aberrant levels of activating transcription factor in the nucleus of melanoma cells are associated with increased metastatic activity of melanoma cells;[54][55][56] studies from mice on skin cancer tend to confirm a role for activating transcription factor-2 in cancer progression.[57][58]
Cancer stem cells may also be involved.[59]
Gene mutations
[edit]Large-scale studies, such as The Cancer Genome Atlas, have characterized recurrent somatic alterations likely driving initiation and development of cutaneous melanoma. The Cancer Genome Atlas study has established four subtypes: BRAF mutant, RAS mutant, NF1 mutant, and triple wild-type.[60]
The most frequent mutation occurs in the 600th codon of BRAF (50% of cases). BRAF is normally involved in cell growth, and this specific mutation renders the protein constitutively active and independent of normal physiological regulation, thus fostering tumor growth.[61] RAS genes (NRAS, HRAS and KRAS) are also recurrently mutated (30% of TCGA cases) and mutations in the 61st or 12th codons trigger oncogenic activity. Loss-of-function mutations often affect tumor suppressor genes such as NF1, TP53 and CDKN2A. Other oncogenic alterations include fusions involving various kinases such as BRAF,[62] RAF1,[63] ALK, RET, ROS1, NTRK1.,[64] NTRK3[65] and MET[66] BRAF, RAS, and NF1 mutations and kinase fusions are remarkably mutually exclusive, as they occur in different subsets of patients. Assessment of mutation status can, therefore, improve patient stratification and inform targeted therapy with specific inhibitors.[citation needed]
In some cases (3–7%) mutated versions of BRAF and NRAS undergo copy-number amplification.[60]
Metastasis
[edit]The research done by Sarna's team proved that heavily pigmented melanoma cells have Young's modulus about 4.93, when in non-pigmented ones it was only 0.98.[67] In another experiment they found that elasticity of melanoma cells is important for its metastasis and growth: non-pigmented tumors were bigger than pigmented and it was much easier for them to spread. They shown that there are both pigmented and non-pigmented cells in melanoma tumors, so that they can both be drug-resistant and metastatic.[67]
Diagnosis
[edit]

Looking at or visually inspecting the area in question is the most common method of suspecting a melanoma.[68] Moles that are irregular in color or shape are typically treated as candidates. To detect melanomas (and increase survival rates), it is recommended to learn to recognize them (see "ABCDE" mnemonic), to regularly examine moles for changes (shape, size, color, itching or bleeding) and to consult a qualified physician when a candidate appears.[69][70] In-person inspection of suspicious skin lesions is more accurate than visual inspection of images of suspicious skin lesions.[71]
When used by trained specialists, dermoscopy is more helpful to identify malignant lesions than use of the naked eye alone.[72] Reflectance confocal microscopy may have better sensitivity and specificity than dermoscopy in diagnosing cutaneous melanoma but more studies are needed to confirm this result.[73]
However, many melanomas present as lesions smaller than 6 mm in diameter, and all melanomas are malignant when they first appear as a small dot. Physicians typically examine all moles, including those less than 6 mm in diameter. Seborrheic keratosis may meet some or all of the ABCD criteria, and can lead to false alarms. Doctors can generally distinguish seborrheic keratosis from melanoma upon examination or with dermatoscopy.[citation needed]
Some advocate replacing "enlarging" with "evolving": moles that change and evolve are a concern. Alternatively, some practitioners prefer "elevation". Elevation can help identify a melanoma, but lack of elevation does not mean that the lesion is not a melanoma. Most melanomas in the US are detected before they become elevated. By the time elevation is visible, they may have progressed to the more dangerous invasive stage.[citation needed]
-
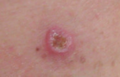
Melanoma in skin biopsy with H&E stain – this case may represent superficial spreading melanoma.
-
Lymph node with almost complete replacement by metastatic melanoma. The brown pigment is a focal deposition of melanin.
-
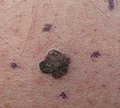
Melanoma, right posterior thigh
-
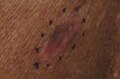
Melanoma in situ, vertex scalp marked for biopsy
-
Melanoma in situ, evolving, right clavicle marked for biopsy
-
Melanoma, vertex scalp marked for biopsy
-
Melanoma, right medial thigh marked for biopsy
-
Melanoma, right posterior shoulder circled for biopsy
-
Melanoma, left forearm marked for biopsy
-
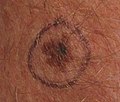
Melanoma left forearm post excision with purse-string closure
-
Melanoma in situ, right forehead marked for biopsy
-
Melanoma in situ, dermatoscope image, right forehead marked for biopsy
-
Melanoma in situ, evolving, a medial right temple with adjacent sebaceous hyperplasia, lateral
-
Melanoma in situ, left anterior shoulder marked for biopsy
-
Melanoma in situ, right anterior shoulder marked for biopsy
-
Melanoma in situ, left upper inner arm
-
Melanoma in situ marked for biopsy, left forearm
-
Melanoma in situ, right upper medial back, marked for biopsy
-
Melanoma, mid frontal scalp
-
Melanoma, left mid-back marked for biopsy
-
Melanoma, left mid-back marked for biopsy, through dermatoscope
-
Gross pathology of melanoma metastasis, which is pigment-forming in a vast majority of cases, giving it a dark appearance
-
Histopathology of a metastatic melanoma to a lymph node, H&E stain, showing poorly differentiated cells
-
Metastatic melanoma on immunohistochemistry for Melan-A, which helps in diagnosing uncertain cases
-
Metastatic melanoma on immunohistochemistry for SOX10, another helpful stain in uncertain cases
Ugly duckling
[edit]One method is the "ugly duckling sign".[74] Correlation of common lesion characteristics is made. Lesions that deviate from the common characteristics are labeled an "ugly duckling", and a further professional exam is required. The "Little Red Riding Hood" sign[74] suggests that individuals with fair skin and light-colored hair might have difficult-to-diagnose amelanotic melanomas. Extra care is required when examining such individuals, as they might have multiple melanomas and severely dysplastic nevi. A dermatoscope must be used to detect "ugly ducklings", as many melanomas in these individuals resemble nonmelanomas or are considered to be "wolves in sheep's clothing".[75]
These fair-skinned individuals often have lightly pigmented or amelanotic melanomas that do not present easy-to-observe color changes and variations. Their borders are often indistinct, complicating visual identification without a dermatoscope.
Amelanotic melanomas and melanomas arising in fair-skinned individuals are very difficult to detect, as they fail to show many of the characteristics in the ABCD rule, break the "ugly duckling" sign, and are hard to distinguish from acne scarring, insect bites, dermatofibromas, or lentigines.
Biopsy
[edit]Following a visual examination and a dermatoscopic exam,[75] or in vivo diagnostic tools such as a confocal microscope, the doctor may biopsy the suspicious mole. A skin biopsy performed under local anesthesia is often required to assist in making or confirming the diagnosis and in defining severity. Elliptical excisional biopsies may remove the tumor, followed by histological analysis and Breslow scoring. Incisional biopsies such as punch biopsies are usually contraindicated in suspected melanomas, because of the possibility of sampling error[76] or local implantation causing misestimation of tumour thickness.[77][78] However, fears that such biopsies may increase the risk of metastatic disease seem unfounded.[79][80]
Total body photography, which involves photographic documentation of as much body surface as possible, is often used during follow-up for high-risk patients. The technique has been reported to enable early detection and provides a cost-effective approach (with any digital camera), but its efficacy has been questioned due to its inability to detect macroscopic changes.[68] The diagnosis method should be used in conjunction with (and not as a replacement for) dermoscopic imaging, with a combination of both methods appearing to give extremely high rates of detection.
Histopathologic types
[edit]Melanoma is a type of neuroectodermal neoplasm.[81] There are four main types of melanoma:[82]
| SN | Type | Features | Incidence[82][notes 1] | Photograph | Micrograph |
|---|---|---|---|---|---|
| 1. | Superficial spreading melanoma | Melanoma cells with nest formation along the dermo-epidermal junction. | 70% | 
|

|
| 2. | Nodular melanoma | Grows relatively more in depth than in width. | 15% - 20% | 
|

|
| 3. | Lentigo maligna melanoma | Linear spread of atypical epidermal melanocytes as well as invasion into the dermis.[83] | 5% - 10% | 
|

|
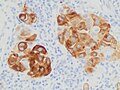
| 4. | Acral lentiginous melanoma | Continuous proliferation of atypical melanocytes at the dermoepidermal junction.[84] | 7% - 10% | 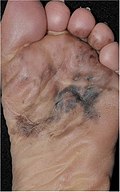
|

|
Other histopathologic types are:

- Mucosal melanoma; When melanoma occurs on mucous membranes.
- Desmoplastic melanoma
- Melanoma with small nevus-like cells
- Melanoma with features of a Spitz nevus
- Uveal melanoma
- Vaginal melanoma
- Polypoid melanoma, a subclass of nodular melanoma.
In situ or invasive
[edit]A melanoma in situ has not invaded beyond the basement membrane, whereas an invasive melanoma has spread beyond it.
Some histopathological types of melanoma are inherently invasive, including nodular melanoma and lentigo maligna melanoma, where the in situ counterpart to lentigo maligna melanoma is lentigo maligna.[85] Lentigo maligna is sometimes classified as a very early melanoma,[86] and sometimes a precursor to melanoma.[87]
Superficial spreading melanomas and acral lentiginous melanomas can be either in situ or invasive,[88] but acral lentiginous melanomas are almost always invasive.[89]
Staging
[edit]Further context on cancer staging is available at TNM.

Metastatic melanomas can be detected by X-rays, CT scans, MRIs, PET and PET/CTs, ultrasound, LDH testing and photoacoustic detection.[90] However, there is lack of evidence in the accuracy of staging of people with melanoma with various imaging methods.[91]
Melanoma stages according to AJCC, 8th edition:[92]
- TX: Primary tumor thickness cannot be assessed (such as a diagnosis by curettage)
- T0: No evidence of primary tumor (such as unknown primary or completely regressed melanoma)
| Stage | T category[92] | Thickness[92] | Ulceration[92] |
|---|---|---|---|
| Stage 0 | Melanoma in situ | ||
| Stage I | T1a | Less than 0.8 mm | No |
| T1b | Less than 0.8 mm | Yes | |
| >0.8 to 1.0 mm | Yes or no | ||
| T2a | >1.0 to 2.0 mm | No | |
| Stage II | T2b | >1.0 to 2.0 mm | Yes |
| T3a | >2.0 to 4.0 mm | No | |
| T3b | >2.0 to 4.0 mm | Yes | |
| T4a | >4.0 mm | No | |
| T4b | >4.0 mm | Yes | |
Stage 1 and 2 require an N (lymph node) class of:
- N0 – No regional metastases.[92]
| Stage | N category | Number of tumor-involved regional lymph nodes | Presence of in-transit, satellite, and/or microsatellite metastases |
|---|---|---|---|
| N/A | NX | Regional nodes not assessed (such as sentinel lymph node biopsy not performed, or regional nodes previously removed for another reason)[notes 2] | |
| Stage III | N1 | One involved lymph node, or any number of in-transit, satellite, and/or microsatellite metastases with no tumor-involved nodes. | |
| N1a | One clinically occult (that is, detected by sentinel node biopsy) | No | |
| N1b | One clinically detected | No | |
| N1c | No regional lymph node disease | Yes | |
| N2 | Two or 3 tumor‐involved nodes or any number of in‐transit, satellite, and/or microsatellite metastases with one tumor‐involved node | ||
| N2a | Two or 3 clinically occult (that is, detected by sentinel node biopsy) | No | |
| N2b | Two or 3, at least one of which was clinically detected | No | |
| N2c | One clinically occult or clinically detected | Yes | |
| N3 | Four or more tumor‐involved nodes or any number of in‐transit, satellite, and/or microsatellite metastases with 2 or more tumor‐involved nodes, or any number of matted nodes without or with in‐transit, satellite, and/or microsatellite metastases | ||
| N3a | Four or more clinically occult (that is, detected by sentinel node biopsy) | No | |
| N3b | Four or more, at least one of which was clinically detected, or the presence of any number of matted nodes | No | |
| N3c | Two or more clinically occult or clinically detected and/or presence of any number of matted nodes | Yes | |
Stage 1, 2 and 3 require an M (metastasis status) of:
- M0: No evidence of distant metastasis
| Stage | M category | Anatomic site | lactate dehydrogenase (LDH) level |
|---|---|---|---|
| Stage IV | M1 | Evidence of distant metastasis | |
| M1a | Distant metastasis to the skin, soft tissue including muscle, and/or non-regional lymph node | Not recorded or unspecified | |
| M1a(0) | Not elevated | ||
| M1a(1) | Elevated | ||
| M1b | Distant metastasis to lung with or without metastasis at M1a sites | Not recorded or unspecified | |
| M1b(0) | Not elevated | ||
| M1b(1) | Elevated | ||
| M1c | Distant metastasis to non‐CNS visceral sites, with or without metastasis to M1a or M1b sites | Not recorded or unspecified | |
| M1c(0) | Not elevated | ||
| M1c(1) | Elevated | ||
| M1d | Distant metastasis to CNS, with or without metastasis to M1a, M1b, or M1c sites | Not recorded or unspecified | |
| M1d(0) | Not elevated | ||
| M1d(1) | Elevated | ||
Older systems include "Clark level" and "Breslow's depth", quantifying microscopic depth of tumor invasion.

Laboratory
[edit]Lactate dehydrogenase (LDH) tests are often used to screen for metastases, although many patients with metastases (even end-stage) have a normal LDH; extraordinarily high LDH often indicates the metastatic spread of the disease to the liver.
It is common for patients diagnosed with melanoma to have chest X-rays and an LDH test, and in some cases CT, MRI, and/or PET scans. Although controversial, sentinel lymph node biopsies and examination of the lymph nodes are also performed in patients to assess spread to the lymph nodes. A diagnosis of melanoma is supported by the presence of the S-100 protein marker.
HMB-45 is a monoclonal antibody that reacts against an antigen present in melanocytic tumors such as melanomas. It is used in anatomic pathology as a marker for such tumors. The antibody was generated to an extract of melanoma. It reacts positively against melanocytic tumors but not other tumors, thus demonstrating specificity and sensitivity. The antibody also reacts positively against junctional nevus cells but not intradermal nevi, and against fetal melanocytes but not normal adult melanocytes.
HMB-45 is nonreactive with almost all non-melanoma human malignancies, with the exception of rare tumors showing evidence of melanogenesis (e.g., pigmented schwannoma, clear cell sarcoma) or tumors associated with tuberous sclerosis complex (angiomyolipoma and lymphangiomyoma).
Prevention
[edit]There is no evidence to support or refute adult population screening for melanoma.[93]
Ultraviolet radiation
[edit]Minimizing exposure to sources of ultraviolet radiation (the sun and sunbeds),[94] following sun protection measures and wearing sun protective clothing (long-sleeved shirts, long trousers, and broad-brimmed hats) can offer protection.
Using artificial light for tanning was once believed to help prevent skin cancers, but it can actually lead to an increased incidence of melanomas.[95]
UV nail lamps, which are used in nail salons to dry nail polish, are another common and widespread source of UV radiation that could be avoided.[96][97] Although the risk of developing skin cancer through UV nail lamp use is low, it is still recommended to wear fingerless gloves and/or apply SPF 30 or greater sunscreen to the hands before using a UV nail lamp.[96][97]
The body uses UV light to generate vitamin D so there is a need to balance getting enough sunlight to maintain healthy vitamin D levels and reducing the risk of melanoma; it takes around a half-hour of sunlight for the body to generate its vitamin D for the day and this is about the same amount of time it takes for fair-skinned people to get a sunburn. Exposure to sunlight can be intermittent instead of all at one time.[98]
Sunscreen
[edit]Sunscreen appears to be effective in preventing melanoma.[2][10] In the past, use of sunscreens with a sun protection factor (SPF) rating of 50 or higher on exposed areas were recommended; as older sunscreens more effectively blocked UVA with higher SPF.[99] Currently, newer sunscreen ingredients (avobenzone, zinc oxide, and titanium dioxide) effectively block both UVA and UVB even at lower SPFs. Sunscreen also protects against squamous cell carcinoma, another skin cancer.[100]
Concerns have been raised that sunscreen might create a false sense of security against sun damage.[101]
Medications
[edit]A 2005 review found tentative evidence that statin and fibrate medication may decrease the risk of melanoma.[102] A 2006 review however did not support any benefit.[103]
Treatment
[edit]
Confirmation of the clinical diagnosis is done with a skin biopsy. This is usually followed up with a wider excision of the scar or tumor. Depending on the stage, a sentinel lymph node biopsy may be performed. Controversy exists around trial evidence for sentinel lymph node biopsy;[104] with unclear evidence of benefit as of 2015.[105] Treatment of advanced melanoma is performed from a multidisciplinary approach.
Surgery
[edit]Excisional biopsies may remove the tumor, but further surgery is often necessary to reduce the risk of recurrence. Complete surgical excision with adequate surgical margins and assessment for the presence of detectable metastatic disease along with short- and long-term followup is standard. Often this is done by a wide local excision (WLE) with 1–2 cm (0.4–0.8 in) margins. Melanoma-in-situ and lentigo malignas are treated with narrower surgical margins, usually 0.2–0.5 cm (0.1–0.2 in). Many surgeons consider 0.5 cm (0.2 in) the standard of care for standard excision of melanoma-in-situ,[106] but 0.2 cm (0.1 in) margin might be acceptable for margin controlled surgery (Mohs surgery, or the double-bladed technique with margin control). The wide excision aims to reduce the rate of tumor recurrence at the site of the original lesion. This is a common pattern of treatment failure in melanoma. Considerable research has aimed to elucidate appropriate margins for excision with a general trend toward less aggressive treatment during the last decades.[107] A 2009 meta-analysis of randomized controlled trials found a small difference in survival rates favoring wide excision of primary cutaneous melanomas, but these results were not statistically significant.[108]
Mohs surgery has been reported with cure rate as low as 77%[109] and as high as 98.0% for melanoma-in-situ.[110] CCPDMA and the "double scalpel" peripheral margin controlled surgery is equivalent to Mohs surgery in effectiveness on this "intra-epithelial" type of melanoma.
Melanomas that spread usually do so to the lymph nodes in the area of the tumor before spreading elsewhere. Attempts to improve survival by removing lymph nodes surgically (lymphadenectomy) were associated with many complications, but no overall survival benefit. Recently, the technique of sentinel lymph node biopsy has been developed to reduce the complications of lymph node surgery while allowing assessment of the involvement of nodes with tumor.[111]
Biopsy of sentinel lymph nodes is a widely used procedure when treating cutaneous melanoma.[112][113]
Neither sentinel lymph node biopsy nor other diagnostic tests should be performed to evaluate early, thin melanoma, including melanoma in situ, T1a melanoma or T1b melanoma ≤ 0.5mm.[114] People with these conditions are unlikely to have the cancer spread to their lymph nodes or anywhere else and have a 5-year survival rate of 97%.[114] Because of these considerations, sentinel lymph node biopsy is considered unnecessary health care for them.[114] Furthermore, baseline blood tests and radiographic studies should not be performed only based on identifying this kind of melanoma, as there are more accurate tests for detecting cancer and these tests have high false-positive rates.[114] To potentially correct false positives, gene expression profiling may be used as auxiliary testing for ambiguous and small lesions.[115][116]
Sentinel lymph node biopsy is often performed, especially for T1b/T2+ tumors, mucosal tumors, ocular melanoma and tumors of the limbs.[citation needed] A process called lymphoscintigraphy is performed in which a radioactive tracer is injected at the tumor site to localize the sentinel node(s). Further precision is provided using a blue tracer dye, and surgery is performed to biopsy the node(s). Routine hematoxylin and eosin (H&E) and immunoperoxidase staining will be adequate to rule out node involvement. Polymerase chain reaction (PCR) tests on nodes, usually performed to test for entry into clinical trials, now demonstrate that many patients with a negative sentinel lymph node actually had a small number of positive cells in their nodes. Alternatively, a fine-needle aspiration biopsy may be performed and is often used to test masses.
If a lymph node is positive, depending on the extent of lymph node spread, a radical lymph node dissection will often be performed. If the disease is completely resected, the patient will be considered for adjuvant therapy. Excisional skin biopsy is the management of choice. Here, the suspect lesion is totally removed with an adequate (but minimal, usually 1 or 2 mm) ellipse of surrounding skin and tissue.[117] To avoid disruption of the local lymphatic drainage, the preferred surgical margin for the initial biopsy should be narrow (1 mm). The biopsy should include the epidermal, dermal, and subcutaneous layers of the skin. This enables the histopathologist to determine the thickness of the melanoma by microscopic examination. This is described by Breslow's thickness (measured in millimeters). However, for large lesions, such as suspected lentigo maligna, or for lesions in surgically difficult areas (face, toes, fingers, eyelids), a small punch biopsy in representative areas will give adequate information and will not disrupt the final staging or depth determination. In no circumstances should the initial biopsy include the final surgical margin (0.5 cm, 1.0 cm, or 2 cm), as a misdiagnosis can result in excessive scarring and morbidity from the procedure. A large initial excision will disrupt the local lymphatic drainage and can affect further lymphangiogram-directed lymphnode dissection. A small punch biopsy can be used at any time where for logistical and personal reasons a patient refuses more invasive excisional biopsy. Small punch biopsies are minimally invasive and heal quickly, usually without noticeable scarring.
Add on treatment
[edit]Adjuvant treatment after surgery may reduce the risk of recurrence after surgery, especially in high-risk melanomas. Routines vary in different countries, but today (2024) the most common adjuvant treatment is immune checkpoint inhibitor treatment for up to a year post-surgery.[118]
In the early 2000s, a relatively common strategy was to treat patients with high risk of recurrence with up to a year of high-dose interferon treatment, which has severe side effects, but may improve the patient's prognosis slightly.[119] A 2013 meta-analysis suggested that the addition of interferon alpha increased disease-free and overall survival for people with AJCC TNM stage II-III cutaneous melanoma.[120] A 2011 meta-analysis showed that interferon could lengthen the time before a melanoma comes back but increased survival by only 3% at 5 years. The unpleasant side effects also greatly decrease quality of life.[121] In the European Union, interferon is usually not used outside the scope of clinical trials.[122][123]
Chemotherapy
[edit]Chemotherapy drugs such as dacarbazine have been the backbone of metastatic melanoma treatment since FDA approval in 1975; however, its efficacy in terms of survival has never been proven in an RCT.[124] Since the approval of immune checkpoint inhibitors, dacarbazine and its oral counterpart temozolomide constitute potential treatment options in later lines of therapy.[125]
Multiple drugs are available to patients to decrease the size of the tumor. By lessening the size of the tumor, some symptoms can be relieved; however, this does not necessarily lead to remission. Some of these drugs are dacarbazine, temozolomide, and fotemustine. Combinations of drugs are also used and, in some cases, present higher remission rates. These medication combinations can have harmful side effects. To maintain quality of life, patients require assistive treatments and observation. Although combinations of drugs increase remission rates, the survival rate does not show an increase.[126]
In people with locally advanced cutaneous malignancies and sarcoma, isolated limb infusion (ILI) has been found to be a minimally invasive and well-tolerated procedure for delivering regional chemotherapy.[127][128]
Targeted therapy
[edit]Melanoma cells have mutations that allow them to survive and grow indefinitely in the body.[124] Small-molecule targeted therapies work by blocking the genes involved in pathways for tumor proliferation and survival.[124] The main treatments are BRAF, C-Kit and NRAS inhibitors.[129] These inhibitors work to inhibit the downstream pathways involved in cell proliferation and tumour development due to specific gene mutations.[130] People can be treated with small-molecule targeted inhibitors if they are positive for the specific mutation.[124] BRAF inhibitors, such as vemurafenib and dabrafenib and a MEK inhibitor trametinib are the most effective, approved treatments for BRAF positive melanoma.[131][124] Melanoma tumors can develop resistance during therapy which can make therapy no longer effective, but combining the use of BRAF and MEK inhibitors may create a fast and lasting melanoma therapy response.[132]
A number of treatments improve survival over traditional chemotherapy.[124] Biochemotherapy (chemotherapy with cytokines IL-2 and IFN-α) combined with BRAF inhibitors improved survival for people with BRAF positive melanoma.[124] Biochemotherapy alone did not improve overall survival and had higher toxicity than chemotherapy.[124] Combining multiple chemotherapy agents (polychemotherapy) did not improve survival over monochemotherapy.[124] Targeted therapies result in relatively short progression-free survival (PFS) times. The therapy combination of dabrafenib and trametinib has a 3-year PFS of 23%, and 5-year PFS of 13%.[133]
Lifileucel (Amtagvi) is a tumor-derived autologous T cell immunotherapy that was approved for medical use in the United States in February 2024.[134][135]
Immunotherapy
[edit]Immunotherapy is aimed at stimulating the person's immune system against the tumor, by enhancing the body's own ability to recognize and kill cancer cells.[136] The current approach to treating melanoma with immunotherapy includes three broad categories of treatments including cytokines, immune check point inhibitors, and adoptive cell transfer.[136] These treatment options are most often used in people with metastatic melanoma and significantly improves overall survival.[124] However, these treatments are often costly. For example, one immune check point inhibitor treatment, pembrolizumab, costs US$10,000 to $12,000 for a single dose administered every 3 weeks.[137]
Cytokine therapies used for melanoma include IFN-a and IL-2.[138] IL-2 (Proleukin) was the first new therapy approved (1990 EU, 1992 US) for the treatment of metastatic melanoma in 20 years.[139] IL-2 may offer the possibility of a complete and long-lasting remission in this disease in a small percentage of people with melanoma.[140] Intralesional IL-2 for in-transit metastases has a high complete response rate ranging from 40 to 100%.[131] Similarly, IFN-a has shown only modest survival benefits and high toxicity, limiting its use as a stand-alone therapy.[124][138]
Immune check point inhibitors include anti-CTLA-4 monoclonal antibodies (ipilimumab and tremelimumab), toll-like receptor (TLR) agonists, CD40 agonists, anti-PD-1 (pembrolizumab, pidilizumab, and nivolumab) and PD-L1 antibodies.[136][138] Evidence suggests that anti-PD-1 antibodies are more effective than anti-CTLA4 antibodies with less systemic toxicity.[124] The five-year progression-free survival for immunotherapy with pembrolizumab is 21%.[133] A therapeutic approach that includes the combination of different therapies improves overall survival and progression-free survival compared to treatment with the separate immunotherapy drugs alone.[124]
Ongoing research is looking at treatment by adoptive cell transfer.[141] Adoptive cell transfer refers to the application of pre-stimulated, modified T cells or dendritic cells and is presently used to minimize complications from graft-versus-host disease.[138][142]
The combination nivolumab/relatlimab (Opdualag) was approved for medical use in the United States in March 2022.[143]
Lentigo maligna
[edit]Standard excision is still being done by most surgeons. Unfortunately, the recurrence rate is exceedingly high (up to 50%). This is due to the ill-defined visible surgical margin, and the facial location of the lesions (often forcing the surgeon to use a narrow surgical margin). The narrow surgical margin used, combined with the limitation of the standard "bread-loafing" technique of fixed tissue histology – result in a high "false negative" error rate, and frequent recurrences. Margin control (peripheral margins) is necessary to eliminate the false negative errors. If bread loafing is used, distances from sections should approach 0.1 mm to assure that the method approaches complete margin control. A meta-analysis of the literature in 2014 found no randomized controlled trials of surgical interventions to treat lentigo maligna or melanoma in-situ, even though surgery is the most widely used treatment.[144]
Mohs surgery has been done with cure rate reported to be as low as 77%,[109] and as high as 95% by another author.[110] The "double scalpel" peripheral margin controlled excision method approximates the Mohs method in margin control, but requires a pathologist intimately familiar with the complexity of managing the vertical margin on the thin peripheral sections and staining methods.[145]
Some melanocytic nevi, and melanoma-in-situ (lentigo maligna) have resolved with an experimental treatment, imiquimod (Aldara) topical cream, an immune enhancing agent. Some derma-surgeons are combining the two methods: surgically excising the cancer and then treating the area with Aldara cream postoperatively for three months. While some studies have suggested the adjuvant use of topical tazarotene, the current evidence is insufficient to recommend it and suggests that it increases topical inflammation, leading to lower patient compliance.[144]
Radiation
[edit]Radiation therapy is often used after surgical resection for patients with locally or regionally advanced melanoma or for patients with un-resectable distant metastases. Kilovoltage x-ray beams are often used for these treatments and have the property of the maximum radiation dose occurring close to the skin surface.[146] It may reduce the rate of local recurrence but does not prolong survival.[147] Radioimmunotherapy of metastatic melanoma is currently under investigation. Radiotherapy has a role in the palliation of metastatic melanoma.[148]
Prognosis
[edit]

Factors that affect prognosis include:
- tumor thickness in millimeters (Breslow's depth),
- depth related to skin structures (Clark level),
- type of melanoma,
- presence of ulceration,
- presence of lymphatic/perineural invasion,
- presence of tumor-infiltrating lymphocytes (if present, prognosis is better),
- location of lesion,
- presence of satellite lesions, and
- presence of regional or distant metastasis.[149]
Certain types of melanoma have worse prognoses but this is explained by their thickness. Less invasive melanomas even with lymph node metastases carry a better prognosis than deep melanomas without regional metastasis at time of staging. Local recurrences tend to behave similarly to a primary unless they are at the site of a wide local excision (as opposed to a staged excision or punch/shave excision) since these recurrences tend to indicate lymphatic invasion.
When melanomas have spread to the lymph nodes, one of the most important factors is the number of nodes with malignancy. Extent of malignancy within a node is also important; micrometastases in which malignancy is only microscopic have a more favorable prognosis than macrometastases. In some cases micrometastases may only be detected by special staining, and if malignancy is only detectable by a rarely employed test known as the polymerase chain reaction (PCR), the prognosis is better. Macro-metastases in which malignancy is clinically apparent (in some cases cancer completely replaces a node) have a far worse prognosis, and if nodes are matted or if there is extracapsular extension, the prognosis is worse still. In addition to these variables, expression levels and copy number variations of a number of relevant genes may be used to support assessment of melanoma prognosis.[115][116]
Stage IV melanoma, in which it has metastasized, is the most deadly skin malignancy: five-year survival is 22.5%.[133] When there is distant metastasis, the cancer is generally considered incurable. The five-year survival rate is less than 10%.[150] The median survival is 6–12 months. Treatment is palliative, focusing on life extension and quality of life. In some cases, patients may live many months or even years with metastatic melanoma (depending on the aggressiveness of the treatment). Metastases to skin and lungs have a better prognosis. Metastases to brain, bone and liver are associated with a worse prognosis. Survival is better with metastasis in which the location of the primary tumor is unknown.[151]
There is not enough definitive evidence to adequately stage, and thus give a prognosis for, ocular melanoma and melanoma of soft parts, or mucosal melanoma (e.g., rectal melanoma), although these tend to metastasize more easily. Even though regression may increase survival, when a melanoma has regressed, it is impossible to know its original size and thus the original tumor is often worse than a pathology report might indicate.
About 200 genes are prognostic in melanoma, with both unfavorable genes where high expression is correlated to poor survival and favorable genes where high expression is associated with longer survival times. Examples of unfavorable genes are MCM6 and TIMELESS; an example of a favorable gene is WIPI1.[51][52]
An increased neutrophil-to-lymphocyte ratio is associated with worse outcomes.[152][153][154]
Epidemiology
[edit]
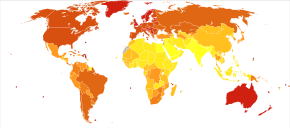
Globally, in 2012, melanoma occurred in 232,000 people and resulted in 55,000 deaths.[2] Australia and New Zealand have the highest rates of melanoma in the world.[2] It has become more common in the last 20 years in areas that are mostly Caucasian.[2]
The rate of melanoma has increased in the recent years, but it is not clear to what extent changes in behavior, in the environment, or in early detection are involved.[156]
Australia
[edit]Australia has a very high – and increasing – rate of melanoma. In 2012, deaths from melanoma occurred in 7.3–9.8 per 100,000 population. In Australia, melanoma is the third most common cancer in either sex; indeed, its incidence is higher than for lung cancer, although the latter accounts for more deaths. It is estimated that in 2012, more than 12,000 Australians were diagnosed with melanoma: given Australia's modest population, this is better expressed as 59.6 new cases per 100,000 population per year; >1 in 10 of all new cancer cases were melanomas.[157] Melanoma incidence in Australia is matter of significance, for the following reasons:
- Australian melanoma incidence has increased by more than 30 per cent between 1991 and 2009.
- Australian melanoma age-standardized incidence rates were, as of 2008, at least 12 times higher than the world average.
- Australian melanoma incidence is, by some margin, the highest in the world.
- Overall age-standardized cancer incidence in Australia is the highest in the world, and this is attributable to melanoma alone. Age-standardized overall cancer incidence is similar to New Zealand, but there is a statistically significant difference between Australia and all other parts of the developed world including North America, Western Europe, and the Mediterranean.
United States
[edit]In the United States, about 9,000 people die from melanoma a year.[159] In 2011, it affected 19.7 per 100,000, and resulted in death in 2.7 per 100,000.[159]
In 2013:
- 71,943 people in the United States were diagnosed with melanomas of the skin, including 42,430 men and 29,513 women.
- 9,394 people in the United States died from melanomas of the skin, including 6,239 men and 3,155 women.[160]
The American Cancer Society's estimates for melanoma incidence in the United States for 2017 are:
- About 87,110 new melanomas will be diagnosed (about 52,170 in men and 34,940 in women).
- About 9,730 people are expected to die of melanoma (about 6,380 men and 3,350 women).
Melanoma is more than 20 times more common in whites than in African Americans. Overall, the lifetime risk of getting melanoma is about 2.5% (1 in 40) for whites, 0.1% (1 in 1,000) for African Americans, and 0.5% (1 in 200) for Mexicans.
The risk of melanoma increases as people age. The average age of people when the disease is diagnosed is 63.[161]
History
[edit]Although melanoma is not a new disease, evidence for its occurrence in antiquity is rather scarce. However, one example lies in a 1960s examination of nine Peruvian mummies, radiocarbon dated to be approximately 2400 years old, which showed apparent signs of melanoma: melanotic masses in the skin and diffuse metastases to the bones.[162]
John Hunter is reported to be the first to operate on metastatic melanoma in 1787. Although not knowing precisely what it was, he described it as a "cancerous fungous excrescence". The excised tumor was preserved in the Hunterian Museum of the Royal College of Surgeons of England. It was not until 1968 that microscopic examination of the specimen revealed it to be an example of metastatic melanoma.[163]
The French physician René Laennec was the first to describe melanoma as a disease entity. His report was initially presented during a lecture for the Faculté de Médecine de Paris in 1804 and then published as a bulletin in 1806.[164]
The first English-language report of melanoma was presented by an English general practitioner from Stourbridge, William Norris in 1820.[165] In his later work in 1857 he remarked that there is a familial predisposition for development of melanoma (Eight Cases of Melanosis with Pathological and Therapeutical Remarks on That Disease). Norris was also a pioneer in suggesting a link between nevi and melanoma and the possibility of a relationship between melanoma and environmental exposures, by observing that most of his patients had pale complexions.[166] He also described that melanomas could be amelanotic and later showed the metastatic nature of melanoma by observing that they can disseminate to other visceral organs.
The first formal acknowledgment of advanced melanoma as untreatable came from Samuel Cooper in 1840. He stated that the only chance for a cure depends upon the early removal of the disease (i.e., early excision of the malignant mole) ...'[167]
More than one and a half centuries later this situation remains largely unchanged.
Terminology
[edit]The word melanoma came to English from 19th-century Neo-Latin[168] and uses combining forms derived from ancient Greek roots: melano- (denoting melanin) + -oma (denoting a tissue mass and especially a neoplasm), in turn from Greek μέλας melas, "dark",[169] and -ωμα oma, "process". The word melanoma has a long history of being used in a broader sense to refer to any melanocytic tumor, typically, but not always malignant,[170][171] but today the narrower sense referring only to malignant types has become so dominant that benign tumors are usually not called melanomas anymore and the word melanoma is now usually taken to mean malignant melanoma unless otherwise specified. Terms such as "benign melanocytic tumor" unequivocally label the benign types, and modern histopathologic tumor classifications used in medicine do not use the word for benign tumors.
Research
[edit]Pharmacotherapy research for un-resectable or metastatic melanoma is ongoing.[172]
Targeted therapies
[edit]In clinical research, adoptive cell therapy and gene therapy, are being tested.[173]
Two kinds of experimental treatments developed at the National Cancer Institute (NCI), have been used in metastatic melanoma with tentative success.[42]
The first treatment involves adoptive cell therapy (ACT) using TILs immune cells (tumor-infiltrating lymphocytes) isolated from a person's own melanoma tumor.[131] These cells are grown in large numbers in a laboratory and returned to the patient after a treatment that temporarily reduces normal T cells in the patient's body. TIL therapy following lymphodepletion can result in durable complete response in a variety of setups.[174][175]
The second treatment, adoptive transfer of genetically altered autologous lymphocytes, depends on delivering genes that encode so called T cell receptors (TCRs), into patient's lymphocytes.[131] After that manipulation lymphocytes recognize and bind to certain molecules found on the surface of melanoma cells and kill them.[176]
A cancer vaccine showed modest benefit in late-stage testing in 2009 against melanoma.[177][178]
BRAF inhibitors
[edit]About 60% of melanomas contain a mutation in the B-Raf gene. Early clinical trials suggested that B-Raf inhibitors including Plexxicon's vemurafenib could lead to substantial tumor regression in a majority of patients if their tumor contain the B-Raf mutation.[179] In June 2011, a large clinical trial confirmed the positive findings from those earlier trials.[180][181]
In August 2011, Vemurafenib received FDA approval for the treatment of late-stage melanoma. In May 2013 the US FDA approved dabrafenib as a single agent treatment for patients with BRAF V600E mutation-positive advanced melanoma.[182]
Some researchers believe that combination therapies that simultaneously block multiple pathways may improve efficacy by making it more difficult for the tumor cells to mutate before being destroyed. In October 2012 a study reported that combining Dabrafenib with a MEK inhibitor trametinib led to even better outcomes. Compared to Dabrafenib alone, progression-free survival was increased to 41% from 9%, and the median progression-free survival increased to 9.4 months versus 5.8 months. Some side effects were, however, increased in the combined study.[183][184]
In January 2014, the FDA approved the combination of dabrafenib and trametinib for the treatment of people with BRAF V600E/K-mutant metastatic melanoma.[185] In June 2018, the FDA approved the combination of a BRAF inhibitor encorafenib and a MEK inhibitor binimetinib for the treatment of un-resectable or metastatic melanoma with a BRAF V600E or V600K mutation.[186]
Eventual resistance to BRAF and MEK inhibitors may be due to a cell surface protein known as EphA2 which is now being investigated.[187]
Ipilimumab
[edit]At the American Society of Clinical Oncology Conference in June 2010, the Bristol Myers Squibb pharmaceutical company reported the clinical findings of their drug ipilimumab. The study found an increase in median survival from 6.4 to 10 months in patients with advanced melanomas treated with the monoclonal ipilimumab, versus an experimental vaccine. It also found a one-year survival rate of 25% in the control group using the vaccine, 44% in the vaccine and ipilimumab group, and 46% in the group treated with ipilimumab alone.[188] However, some have raised concerns about this study for its use of the unconventional control arm, rather than comparing the drug against a placebo or standard treatment.[189][190] The criticism was that although Ipilimumab performed better than the vaccine, the vaccine has not been tested before and may be causing toxicity, making the drug appear better by comparison.
Ipilimumab was approved by the FDA in March 2011 to treat patients with late-stage melanoma that has spread or cannot be removed by surgery.[191][192][193]
In June 2011, a clinical trial of ipilimumab plus dacarbazine combined this immune system booster with the standard chemotherapy drug that targets cell division. It showed an increase in median survival for these late stage patients to 11 months instead of the 9 months normally seen. Researchers were also hopeful of improving the five year survival rate, though serious adverse side-effects were seen in some patients. A course of treatment costs $120,000. The drug's brandname is Yervoy.[180][194]
Surveillance methods
[edit]Advances in high resolution ultrasound scanning have enabled surveillance of metastatic burden to the sentinel lymph nodes.[195] The Screening and Surveillance of Ultrasound in Melanoma trial (SUNMEL) is evaluating ultrasound as an alternative to invasive surgical methods.[196]
Oncolytic virotherapy
[edit]In some countries oncolytic virotherapy methods are studied and used to treat melanoma. Oncolytic virotherapy is a promising branch of virotherapy, where oncolytic viruses are used to treat diseases; viruses can increase metabolism, reduce anti-tumor immunity and disorganize vasculature.[197] Talimogene laherparepvec (T-VEC) (which is a herpes simplex virus type 1–derived oncolytic immunotherapy), was shown to be useful against metastatic melanoma in 2015 with an increased survival of 4.4 months.[198][12]
Antivirals
[edit]Antiretrovirals have been tested in vitro against melanoma. The rationale behind this lies in their potential to inhibit human endogenous retroviruses, whose activity has been associated with the development of melanoma.[199][200] The results on malignant cells have shown moderate efficacy, and further developments are awaited through animal model testing.
Notes
[edit]References
[edit]- ^ a b c d e f g h i j k l m "Melanoma Treatment – for health professionals". National Cancer Institute. 26 June 2015. Archived from the original on 4 July 2015. Retrieved 30 June 2015.
- ^ a b c d e f g h i j k l m n o p q r World Cancer Report (PDF). World Health Organization. 2014. pp. Chapter 5.14. ISBN 978-92-832-0429-9. Archived (PDF) from the original on 30 May 2014.
- ^ Goldstein BG, Goldstein AO (April 2001). "Diagnosis and management of malignant melanoma". American Family Physician. 63 (7): 1359–68, 1374. PMID 11310650.
- ^ a b "SEER Stat Fact Sheets: Melanoma of the Skin". NCI. Archived from the original on 6 July 2014.
- ^ a b Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease Injury Incidence Prevalence Collaborators) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ a b Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ Schwartzman RM, Orkin M (1962). A Comparative Study of Diseases of Dog and Man. Springfield, IL: Thomas. p. 85.
The term 'melanoma' in human medicine indicates a malignant growth; the prefix 'malignant' is redundant.
- ^ Bobonich M, Nolen ME (2015). Dermatology for Advanced Practice Clinicians. Philadelphia: Wolters Kluwer. p. 106.
The term malignant melanoma is becoming obsolete because the word 'malignant' is redundant as there are no benign melanomas.
- ^ Farlex Partner Medical Dictionary. 2012. Archived from the original on 10 June 2022. Retrieved 4 March 2021.
Avoid the redundant phrase malignant melanoma.
- ^ a b Kanavy HE, Gerstenblith MR (December 2011). "Ultraviolet radiation and melanoma". Seminars in Cutaneous Medicine and Surgery. 30 (4): 222–228. doi:10.1016/j.sder.2011.08.003 (inactive 1 November 2024). PMID 22123420.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ a b Azoury SC, Lange JR (October 2014). "Epidemiology, risk factors, prevention, and early detection of melanoma". The Surgical Clinics of North America. 94 (5): 945–62, vii. doi:10.1016/j.suc.2014.07.013. PMID 25245960.
- ^ a b Syn NL, Teng MW, Mok TS, Soo RA (December 2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet. Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- ^ "USCS Data Visualizations". gis.cdc.gov. Archived from the original on 17 March 2020. Retrieved 7 March 2020.
Need to select "melanoma"
- ^ "CDC - What Are the Symptoms of Skin Cancer?". www.cdc.gov. 26 June 2018. Archived from the original on 7 December 2018. Retrieved 1 February 2019.
- ^ Daniel Jensen J, Elewski BE (February 2015). "The ABCDEF Rule: Combining the "ABCDE Rule" and the "Ugly Duckling Sign" in an Effort to Improve Patient Self-Screening Examinations". The Journal of Clinical and Aesthetic Dermatology. 8 (2): 15. PMC 4345927. PMID 25741397.
- ^ "The EFG of Nodular Melanomas | MoleMap New Zealand". The EFG of Nodular Melanomas | MoleMap New Zealand. Archived from the original on 2 February 2019. Retrieved 1 February 2019.
- ^ Fiddler IJ (October 1995). "Melanoma Metastasis". Cancer Control. 2 (5): 398–404. doi:10.1177/107327489500200503. PMID 10862180.
- ^ a b c d e f "Melanoma Risk factors". Mayo Clinic. Archived from the original on 10 April 2017. Retrieved 10 April 2017.
- ^ a b Greene MH (December 1999). "The genetics of hereditary melanoma and nevi. 1998 update". Cancer. 86 (11 Suppl): 2464–2477. doi:10.1002/(SICI)1097-0142(19991201)86:11+<2464::AID-CNCR3>3.0.CO;2-F. PMID 10630172. S2CID 32817426.
- ^ a b c Goydos JS, Shoen SL (2016). "Acral Lentiginous Melanoma". Melanoma. Cancer Treatment and Research. Vol. 167. pp. 321–9. doi:10.1007/978-3-319-22539-5_14. ISBN 978-3-319-22538-8. PMID 26601870.
- ^ Perkins A, Duffy RL (June 2015). "Atypical moles: diagnosis and management". American Family Physician. 91 (11): 762–767. PMID 26034853.
- ^ Boniol M, Autier P, Boyle P, Gandini S (July 2012). "Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis". BMJ. 345: e4757. doi:10.1136/bmj.e4757. PMC 3404185. PMID 22833605.
- ^ El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. (WHO International Agency for Research on Cancer Monograph Working Group) (August 2009). "A review of human carcinogens--part D: radiation". The Lancet. Oncology. 10 (8): 751–752. doi:10.1016/S1470-2045(09)70213-X. PMID 19655431.
- ^ Sanlorenzo M, Wehner MR, Linos E, Kornak J, Kainz W, Posch C, et al. (January 2015). "The risk of melanoma in airline pilots and cabin crew: a meta-analysis". JAMA Dermatology. 151 (1): 51–58. doi:10.1001/jamadermatol.2014.1077. PMC 4482339. PMID 25188246.
- ^ Rünger TM, Farahvash B, Hatvani Z, Rees A (January 2012). "Comparison of DNA damage responses following equimutagenic doses of UVA and UVB: a less effective cell cycle arrest with UVA may render UVA-induced pyrimidine dimers more mutagenic than UVB-induced ones". Photochemical & Photobiological Sciences. 11 (1): 207–215. doi:10.1039/c1pp05232b. PMID 22005748. S2CID 25209863.
- ^ Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, et al. (May 2001). "Ultraviolet A and melanoma: a review". Journal of the American Academy of Dermatology. 44 (5): 837–846. doi:10.1067/mjd.2001.114594. PMID 11312434. S2CID 7655216.
- ^ Oliveria SA, Saraiya M, Geller AC, Heneghan MK, Jorgensen C (February 2006). "Sun exposure and risk of melanoma". Archives of Disease in Childhood. 91 (2): 131–138. doi:10.1136/adc.2005.086918. PMC 2082713. PMID 16326797.
- ^ Lee JA, Strickland D (May 1980). "Malignant melanoma: social status and outdoor work". British Journal of Cancer. 41 (5): 757–763. doi:10.1038/bjc.1980.138. PMC 2010319. PMID 7426301.
- ^ Pion IA, Rigel DS, Garfinkel L, Silverman MK, Kopf AW (January 1995). "Occupation and the risk of malignant melanoma". Cancer. 75 (2 Suppl): 637–644. doi:10.1002/1097-0142(19950115)75:2+<637::aid-cncr2820751404>3.0.co;2-#. PMID 7804988. S2CID 196354681.
- ^ "WHO | The World Health Organization recommends that no person under 18 should use a sunbed". WHO. Archived from the original on 16 June 2009.
- ^ Khlat M, Vail A, Parkin M, Green A (May 1992). "Mortality from melanoma in migrants to Australia: variation by age at arrival and duration of stay". American Journal of Epidemiology. 135 (10): 1103–1113. doi:10.1093/oxfordjournals.aje.a116210. PMID 1632422.
- ^ "Can we get skin cancer from tanning beds?". CuradermBCC. 1 September 2022. Archived from the original on 3 September 2022. Retrieved 3 September 2022.
- ^ Soura E, Eliades PJ, Shannon K, Stratigos AJ, Tsao H (March 2016). "Hereditary melanoma: Update on syndromes and management: Genetics of familial atypical multiple mole melanoma syndrome". Journal of the American Academy of Dermatology. 74 (3): 395–407, quiz 408–410. doi:10.1016/j.jaad.2015.08.038. PMC 4761105. PMID 26892650.
- ^ Halachmi S, Gilchrest BA (March 2001). "Update on genetic events in the pathogenesis of melanoma". Current Opinion in Oncology. 13 (2): 129–136. doi:10.1097/00001622-200103000-00008. PMID 11224711. S2CID 29876528.
- ^ "CDKN2A cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4)". U.S. National Library of Medicine. Archived from the original on 17 November 2004. Retrieved 4 September 2017.
- ^ Soura E, Eliades PJ, Shannon K, Stratigos AJ, Tsao H (March 2016). "Hereditary melanoma: Update on syndromes and management: Genetics of familial atypical multiple mole melanoma syndrome". Journal of the American Academy of Dermatology. 74 (3): 395–407, quiz 408–10. doi:10.1016/j.jaad.2015.08.038. PMC 4761105. PMID 26892650.
- ^ Firoz EF, Warycha M, Zakrzewski J, Pollens D, Wang G, Shapiro R, et al. (April 2009). "Association of MDM2 SNP309, age of onset, and gender in cutaneous melanoma". Clinical Cancer Research. 15 (7): 2573–2580. doi:10.1158/1078-0432.CCR-08-2678. PMC 3881546. PMID 19318491.
- ^ Bliss JM, Ford D, Swerdlow AJ, Armstrong BK, Cristofolini M, Elwood JM, et al. (August 1995). "Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. The International Melanoma Analysis Group (IMAGE)". International Journal of Cancer. 62 (4): 367–376. doi:10.1002/ijc.2910620402. PMID 7635560. S2CID 9539601.
- ^ Miller AJ, Mihm MC (July 2006). "Melanoma". The New England Journal of Medicine. 355 (1): 51–65. doi:10.1056/NEJMra052166. PMID 16822996.
- ^ Rhodes AR, Weinstock MA, Fitzpatrick TB, Mihm MC, Sober AJ (December 1987). "Risk factors for cutaneous melanoma. A practical method of recognizing predisposed individuals". JAMA. 258 (21): 3146–3154. doi:10.1001/jama.258.21.3146. PMID 3312689.
- ^ Ciarletta P, Foret L, Ben Amar M (March 2011). "The radial growth phase of malignant melanoma: multi-phase modelling, numerical simulations and linear stability analysis". Journal of the Royal Society, Interface. 8 (56): 345–368. doi:10.1098/rsif.2010.0285. PMC 3030817. PMID 20656740.
- ^ a b Hershkovitz L, Schachter J, Treves AJ, Besser MJ (2010). "Focus on adoptive T cell transfer trials in melanoma". Clinical & Developmental Immunology. 2010: 260267. doi:10.1155/2010/260267. PMC 3018069. PMID 21234353.
- ^ Spatz A, Gimotty PA, Cook MG, van den Oord JJ, Desai N, Eggermont AM, et al. (2007). "ASCO Annual Meeting Proceedings Part I. Abstract: Protective effect of a brisk tumor infiltrating lymphocyte infiltrate in melanoma: An EORTC melanoma group study". Journal of Clinical Oncology. 25 (18S): 8519. doi:10.1200/jco.2007.25.18_suppl.8519. Archived from the original on 25 July 2011.
- ^ Davies MA, Samuels Y (October 2010). "Analysis of the genome to personalize therapy for melanoma". Oncogene. 29 (41): 5545–5555. doi:10.1038/onc.2010.323. PMC 3169242. PMID 20697348.
- ^ Barrett JC (1991). "Mutagenesis and Carcinogenesis". In Brugge J, Curran T, Harlow E, McCormick F (eds.). Origins of Human Cancer. Cold Spring Harbor Press. ISBN 0-87969-404-1 – via Internet Archive.
- ^ Sage E, Girard PM, Francesconi S (January 2012). "Unravelling UVA-induced mutagenesis". Photochemical & Photobiological Sciences. 11 (1): 74–80. doi:10.1039/c1pp05219e. PMID 21901217. S2CID 45189513.
- ^ Budden T, Bowden NA (January 2013). "The role of altered nucleotide excision repair and UVB-induced DNA damage in melanomagenesis". International Journal of Molecular Sciences. 14 (1): 1132–1151. doi:10.3390/ijms14011132. PMC 3565312. PMID 23303275.
- ^ Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. (May 2012). "Melanoma genome sequencing reveals frequent PREX2 mutations". Nature. 485 (7399): 502–506. Bibcode:2012Natur.485..502B. doi:10.1038/nature11071. PMC 3367798. PMID 22622578.
- ^ Roach JC, Glusman G, Smit AF, Huff CD, Hubley R, Shannon PT, et al. (April 2010). "Analysis of genetic inheritance in a family quartet by whole-genome sequencing". Science. 328 (5978): 636–639. Bibcode:2010Sci...328..636R. doi:10.1126/science.1186802. PMC 3037280. PMID 20220176.
- ^ Campbell CD, Chong JX, Malig M, Ko A, Dumont BL, Han L, et al. (November 2012). "Estimating the human mutation rate using autozygosity in a founder population". Nature Genetics. 44 (11): 1277–1281. doi:10.1038/ng.2418. PMC 3483378. PMID 23001126.
- ^ a b "The human pathology proteome in melanoma – The Human Protein Atlas". www.proteinatlas.org. Archived from the original on 2 October 2017. Retrieved 2 October 2017.
- ^ a b Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. (August 2017). "A pathology atlas of the human cancer transcriptome". Science. 357 (6352): eaan2507. doi:10.1126/science.aan2507. PMID 28818916. S2CID 206659235.
- ^ Ellrott K, Bailey MH, Saksena G, Covington KR, Kandoth C, Stewart C, et al. (March 2018). "Scalable Open Science Approach for Mutation Calling of Tumor Exomes Using Multiple Genomic Pipelines". Cell Systems. 6 (3): 271–281.e7. doi:10.1016/j.cels.2018.03.002. PMC 6075717. PMID 29596782.
- ^ Leslie MC, Bar-Eli M (January 2005). "Regulation of gene expression in melanoma: new approaches for treatment". Journal of Cellular Biochemistry. 94 (1): 25–38. doi:10.1002/jcb.20296. PMID 15523674. S2CID 23515325.
- ^ Bhoumik A, Singha N, O'Connell MJ, Ronai ZA (June 2008). "Regulation of TIP60 by ATF2 modulates ATM activation". The Journal of Biological Chemistry. 283 (25): 17605–17614. doi:10.1074/jbc.M802030200. PMC 2427333. PMID 18397884.
- ^ Bhoumik A, Jones N, Ronai Z (March 2004). "Transcriptional switch by activating transcription factor 2-derived peptide sensitizes melanoma cells to apoptosis and inhibits their tumorigenicity". Proceedings of the National Academy of Sciences of the United States of America. 101 (12): 4222–4227. Bibcode:2004PNAS..101.4222B. doi:10.1073/pnas.0400195101. PMC 384722. PMID 15010535.
- ^ Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V (April 2008). "The role of ATF-2 in oncogenesis". BioEssays. 30 (4): 314–327. doi:10.1002/bies.20734. PMID 18348191. S2CID 678541.
- ^ Huang Y, Minigh J, Miles S, Niles RM (February 2008). "Retinoic acid decreases ATF-2 phosphorylation and sensitizes melanoma cells to taxol-mediated growth inhibition". Journal of Molecular Signaling. 3: 3. doi:10.1186/1750-2187-3-3. PMC 2265711. PMID 18269766.
- ^ Parmiani G (March 2016). "Melanoma Cancer Stem Cells: Markers and Functions". Cancers. 8 (3): 34. doi:10.3390/cancers8030034. PMC 4810118. PMID 26978405.
- ^ a b Akbani R, Akdemir KC, Aksoy BA, Albert M, Ally A, Amin SB, et al. (Cancer Genome Atlas Network) (June 2015). "Genomic Classification of Cutaneous Melanoma". Cell. 161 (7): 1681–1696. doi:10.1016/j.cell.2015.05.044. PMC 4580370. PMID 26091043.
- ^ Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, et al. (July 2012). "The role of BRAF V600 mutation in melanoma". Journal of Translational Medicine. 10 (1): 85. doi:10.1186/1479-5876-10-85. PMC 3391993. PMID 22554099.
- ^ Botton T, Talevich E, Mishra VK, Zhang T, Shain AH, Berquet C, et al. (October 2019). "Genetic Heterogeneity of BRAF Fusion Kinases in Melanoma Affects Drug Responses". Cell Reports. 29 (3): 573–588.e7. doi:10.1016/j.celrep.2019.09.009. PMC 6939448. PMID 31618628.
- ^ McEvoy CR, Xu H, Smith K, Etemadmoghadam D, San Leong H, Choong DY, et al. (May 2019). "Profound MEK inhibitor response in a cutaneous melanoma harboring a GOLGA4-RAF1 fusion". The Journal of Clinical Investigation. 129 (5): 1940–1945. doi:10.1172/JCI123089. PMC 6486352. PMID 30835257.
- ^ Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. (May 2014). "Kinase fusions are frequent in Spitz tumours and spitzoid melanomas". Nature Communications. 5 (1): 3116. Bibcode:2014NatCo...5.3116W. doi:10.1038/ncomms4116. PMC 4084638. PMID 24445538.
- ^ Yeh I, Tee MK, Botton T, Shain AH, Sparatta AJ, Gagnon A, et al. (November 2016). "NTRK3 kinase fusions in Spitz tumours". The Journal of Pathology. 240 (3): 282–290. doi:10.1002/path.4775. PMC 5071153. PMID 27477320.
- ^ Yeh I, Botton T, Talevich E, Shain AH, Sparatta AJ, de la Fouchardiere A, et al. (May 2015). "Activating MET kinase rearrangements in melanoma and Spitz tumours". Nature Communications. 6 (1): 7174. Bibcode:2015NatCo...6.7174Y. doi:10.1038/ncomms8174. PMC 4446791. PMID 26013381.
- ^ a b Sarna M, Krzykawska-Serda M, Jakubowska M, Zadlo A, Urbanska K (June 2019). "Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion". Scientific Reports. 9 (1): 9280. Bibcode:2019NatSR...9.9280S. doi:10.1038/s41598-019-45643-9. PMC 6594928. PMID 31243305.
- ^ a b Wurm EM, Soyer HP (October 2010). "Scanning for melanoma". Australian Prescriber. 33: 150–55. doi:10.18773/austprescr.2010.070.
- ^ "Prevention: ABCD's of Melanoma". American Melanoma Foundation. Archived from the original on 23 April 2003.
- ^ Friedman RJ, Rigel DS, Kopf AW (1985). "Early detection of malignant melanoma: the role of physician examination and self-examination of the skin". CA. 35 (3): 130–151. doi:10.3322/canjclin.35.3.130. PMID 3921200. S2CID 20787489.
- ^ Dinnes J, Deeks JJ, Grainge MJ, Chuchu N, Ferrante di Ruffano L, Matin RN, et al. (December 2018). "Visual inspection for diagnosing cutaneous melanoma in adults". The Cochrane Database of Systematic Reviews. 2018 (12): CD013194. doi:10.1002/14651858.CD013194. PMC 6492463. PMID 30521684.
- ^ Dinnes J, Deeks JJ, Chuchu N, Ferrante di Ruffano L, Matin RN, Thomson DR, et al. (December 2018). "Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults". The Cochrane Database of Systematic Reviews. 2018 (12): CD011902. doi:10.1002/14651858.CD011902.pub2. PMC 6517096. PMID 30521682.
- ^ Dinnes J, Deeks JJ, Saleh D, Chuchu N, Bayliss SE, Patel L, et al. (Cochrane Skin Group) (December 2018). "Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults". The Cochrane Database of Systematic Reviews. 12 (12): CD013190. doi:10.1002/14651858.CD013190. PMC 6492459. PMID 30521681.
- ^ a b Mascaro JM, Mascaro JM (November 1998). "The dermatologist's position concerning nevi: a vision ranging from "the ugly duckling" to "little red riding hood"". Archives of Dermatology. 134 (11): 1484–1485. doi:10.1001/archderm.134.11.1484. PMID 9828892.
- ^ a b "Introduction to Dermoscopy". DermNet New Zealand. Archived from the original on 7 May 2009.
- ^ Montgomery BD, Sadler GM (January 2009). "Punch biopsy of pigmented lesions is potentially hazardous". Canadian Family Physician. 55 (1): 24, autor reply 24. PMC 2628830. PMID 19155361.
- ^ Luk PP, Vilain R, Crainic O, McCarthy SW, Thompson JF, Scolyer RA (August 2015). "Punch biopsy of melanoma causing tumour cell implantation: another peril of utilising partial biopsies for melanocytic tumours". The Australasian Journal of Dermatology. 56 (3): 227–231. doi:10.1111/ajd.12333. PMID 25827527. S2CID 2510803.
- ^ Lin SW, Kaye V, Goldfarb N, Rawal A, Warshaw E (July 2012). "Melanoma tumor seeding after punch biopsy". Dermatologic Surgery. 38 (7 Pt 1): 1083–1085. doi:10.1111/j.1524-4725.2012.02384.x. PMID 22471244. S2CID 3431248.
- ^ Martin RC, Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Edwards MJ, et al. (December 2005). "Is incisional biopsy of melanoma harmful?". American Journal of Surgery. 190 (6): 913–917. doi:10.1016/j.amjsurg.2005.08.020. PMID 16307945.
- ^ Yamashita Y, Hashimoto I, Abe Y, Seike T, Okawa K, Senzaki Y, et al. (March 2014). "Effect of biopsy technique on the survival rate of malignant melanoma patients". Archives of Plastic Surgery. 41 (2): 122–125. doi:10.5999/aps.2014.41.2.122. PMC 3961608. PMID 24665419.
- ^ Mills SE (March 2002). "Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas". Modern Pathology. 15 (3): 264–278. doi:10.1038/modpathol.3880522. PMID 11904342. S2CID 34498802.
- ^ a b Ferri F (2019). "Melanoma". Ferri's clinical advisor 2019 : 5 books in 1. Philadelphia, PA: Elsevier. p. 805. ISBN 978-0-323-52957-0. OCLC 1040695302. Archived from the original on 4 August 2020.
- ^ Xiong M, Charifa A, Chen CS (2020). "Lentigo Maligna Melanoma". Cancer, Lentigo Maligna Melanoma. StatPearls. PMID 29489150. Archived from the original on 8 March 2021. Retrieved 13 February 2020 – via National Center for Biotechnology Information. Last Update: 18 May 2019.
- ^ Piliang MP (September 2009). "Acral Lentiginous Melanoma". Surgical Pathology Clinics. 2 (3): 535–541. doi:10.1016/j.path.2009.08.005. PMID 26838538.
- ^ McKenna JK, Florell SR, Goldman GD, Bowen GM (April 2006). "Lentigo maligna/lentigo maligna melanoma: current state of diagnosis and treatment". Dermatologic Surgery. 32 (4): 493–504. doi:10.1111/j.1524-4725.2006.32102.x. PMID 16681656. S2CID 8312676.
- ^ "Precancerous conditions of the skin". Canadian Cancer Society. Archived from the original on 3 August 2020. Retrieved 26 February 2020.
- ^ Fleming C (2010). "How to manage patients with lentigo maligna". Melanoma Research. 20: e26. doi:10.1097/01.cmr.0000382797.99333.66. ISSN 0960-8931. S2CID 71464540.
- ^ Hale CS. "Skin melanocytic tumor - Melanoma - Melanoma in situ". pathology Outlines. Archived from the original on 26 February 2020. Retrieved 26 February 2020. Topic Completed: 1 May 2013. Revised: 23 May 2019
- ^ Park HS, Cho KH (April 2010). "Acral lentiginous melanoma in situ: a diagnostic and management challenge". Cancers. 2 (2): 642–652. doi:10.3390/cancers2020642. PMC 3835096. PMID 24281086.
- ^ Weight RM, Viator JA, Dale PS, Caldwell CW, Lisle AE (October 2006). "Photoacoustic detection of metastatic melanoma cells in the human circulatory system". Optics Letters. 31 (20): 2998–3000. Bibcode:2006OptL...31.2998W. doi:10.1364/OL.31.002998. PMID 17001379.
- ^ Dinnes J, Ferrante di Ruffano L, Takwoingi Y, Cheung ST, Nathan P, Matin RN, et al. (Cochrane Skin Group) (July 2019). "Ultrasound, CT, MRI, or PET-CT for staging and re-staging of adults with cutaneous melanoma". The Cochrane Database of Systematic Reviews. 2019 (7): CD012806. doi:10.1002/14651858.CD012806.pub2. PMC 6601698. PMID 31260100.
- ^ a b c d e Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. (November 2017). "Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual". CA. 67 (6): 472–492. doi:10.3322/caac.21409. PMC 5978683. PMID 29028110., citing
Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York: Springer International Publishing; 2017:563‐585). - ^ Johansson M, Brodersen J, Gøtzsche PC, Jørgensen KJ, et al. (Cochrane Skin Group) (June 2019). "Screening for reducing morbidity and mortality in malignant melanoma". The Cochrane Database of Systematic Reviews. 2019 (6): CD012352. doi:10.1002/14651858.CD012352.pub2. PMC 6545529. PMID 31157404.
- ^ Autier P (October 2005). "Cutaneous malignant melanoma: facts about sunbeds and sunscreen". Expert Review of Anticancer Therapy. 5 (5): 821–833. doi:10.1586/14737140.5.5.821. PMID 16221052. S2CID 6091309.
- ^ Clough-Gorr KM, Titus-Ernstoff L, Perry AE, Spencer SK, Ernstoff MS (September 2008). "Exposure to sunlamps, tanning beds, and melanoma risk". Cancer Causes & Control. 19 (7): 659–669. doi:10.1007/s10552-008-9129-6. PMC 6367654. PMID 18273687.
- ^ a b Shihab N, Lim HW (November 2018). "Potential cutaneous carcinogenic risk of exposure to UV nail lamp: A review". Photodermatology, Photoimmunology & Photomedicine. 34 (6): 362–365. doi:10.1111/phpp.12398. PMID 29882991. S2CID 46958616.
- ^ a b O'Sullivan NA, Tait CP (May 2014). "Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature". The Australasian Journal of Dermatology. 55 (2): 99–106. doi:10.1111/ajd.12145. PMID 24592921. S2CID 206984768.
- ^ Greinert R, de Vries E, Erdmann F, Espina C, Auvinen A, Kesminiene A, et al. (December 2015). "European Code against Cancer 4th Edition: Ultraviolet radiation and cancer". Cancer Epidemiology. 39 (Suppl 1): S75–S83. doi:10.1016/j.canep.2014.12.014. hdl:1765/86460. PMID 26096748.
- ^ "Can Melanoma Be Prevented?". Archived from the original on 27 June 2006.
- ^ Burnett ME, Wang SQ (April 2011). "Current sunscreen controversies: a critical review". Photodermatology, Photoimmunology & Photomedicine. 27 (2): 58–67. doi:10.1111/j.1600-0781.2011.00557.x. PMID 21392107. S2CID 29173997.
- ^ Planta MB (1 November 2011). "Sunscreen and melanoma: is our prevention message correct?". Journal of the American Board of Family Medicine. 24 (6): 735–739. doi:10.3122/jabfm.2011.06.100178. PMID 22086817. S2CID 1477485.
- ^ Dellavalle RP, Drake A, Graber M, Heilig LF, Hester EJ, Johnson KR, et al. (October 2005). "Statins and fibrates for preventing melanoma". The Cochrane Database of Systematic Reviews. 2014 (4): CD003697. doi:10.1002/14651858.CD003697.pub2. PMC 11102950. PMID 16235336. Archived from the original on 4 July 2018. Retrieved 3 July 2018.
- ^ Freeman SR, Drake AL, Heilig LF, Graber M, McNealy K, Schilling LM, et al. (November 2006). "Statins, fibrates, and melanoma risk: a systematic review and meta-analysis". Journal of the National Cancer Institute. 98 (21): 1538–1546. doi:10.1093/jnci/djj412. PMID 17077356.
- ^ "The Sentinel Node Biopsy Procedure in Melanoma does not offer a survival advantage". Malignant Melanoma. 8 January 2008. Archived from the original on 11 July 2012. Retrieved 13 August 2012.
- ^ Kyrgidis A, Tzellos T, Mocellin S, Apalla Z, Lallas A, Pilati P, et al. (May 2015). "Sentinel lymph node biopsy followed by lymph node dissection for localised primary cutaneous melanoma". The Cochrane Database of Systematic Reviews. 2015 (5): CD010307. doi:10.1002/14651858.CD010307.pub2. PMC 6461196. PMID 25978975.
- ^ Clark GS, Pappas-Politis EC, Cherpelis BS, Messina JL, Möller MG, Cruse CW, et al. (July 2008). "Surgical management of melanoma in situ on chronically sun-damaged skin". Cancer Control. 15 (3): 216–224. doi:10.1177/107327480801500304. PMID 18596673.
- ^ Balch CM, Urist MM, Karakousis CP, Smith TJ, Temple WJ, Drzewiecki K, et al. (September 1993). "Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial". Annals of Surgery. 218 (3): 262–7, discussion 267–9. doi:10.1097/00000658-199309000-00005. PMC 1242959. PMID 8373269.
- ^ Sladden MJ, Balch C, Barzilai DA, Berg D, Freiman A, Handiside T, et al. (October 2009). "Surgical excision margins for primary cutaneous melanoma". The Cochrane Database of Systematic Reviews. 129 (4): CD004835. doi:10.1002/14651858.CD004835.pub2. PMID 19821334.
- ^ a b Mohs FE, Mikhail GR (January 1991). Mohs micrographic surgery. W.B. Saunders. pp. 13–14. ISBN 978-0-7216-3415-9. Archived from the original on 7 January 2016.
- ^ a b Bene NI, Healy C, Coldiron BM (May 2008). "Mohs micrographic surgery is accurate 95.1% of the time for melanoma in situ: a prospective study of 167 cases". Dermatologic Surgery. 34 (5): 660–664. doi:10.1111/j.1524-4725.2007.34124.x. PMID 18261099. S2CID 23386371.
Cure rate as high as 98% for small melanoma in situ, and as high as 95% noted for lentigo maligna variant of melanona in situ has been reported with Mohs surgery.
- ^ "The Screening and Surveillance of Ultrasound in Melanoma trial (SUNMEL)". Archived from the original on 6 January 2009.
- ^ Crowson AN, Haskell H (October 2013). "The role of sentinel lymph-node biopsy in the management of cutaneous melanoma". Giornale Italiano di Dermatologia e Venereologia. 148 (5): 493–499. PMID 24005142.
- ^ Ross MI, Gershenwald JE (May–June 2013). "Sentinel lymph node biopsy for melanoma: a critical update for dermatologists after two decades of experience". Clinics in Dermatology. 31 (3): 298–310. doi:10.1016/j.clindermatol.2012.08.004. PMID 23608449.
- ^ a b c d American Academy of Dermatology (February 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Academy of Dermatology, archived from the original on 1 December 2013, retrieved 5 December 2013, which cites:
- Bichakjian CK, Halpern AC, Johnson TM, Foote Hood A, Grichnik JM, Swetter SM, et al. (American Academy of Dermatology) (November 2011). "Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology". Journal of the American Academy of Dermatology. 65 (5): 1032–1047. doi:10.1016/j.jaad.2011.04.031. hdl:20.500.12749/1682. PMID 21868127.
- American Joint Committee on Cancer (2010). Edge SB (ed.). AJCC cancer staging manual (7th ed.). Springer. ISBN 978-0-387-88440-0.
- National Comprehensive Cancer Network (2012), National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines): melanoma (PDF), Fort Washington, Pennsylvania: National Comprehensive Cancer Network, archived from the original (PDF) on 28 December 2013, retrieved 5 December 2013

- ^ a b van Kempen LC, Redpath M, Robert C, Spatz A (November 2014). "Molecular pathology of cutaneous melanoma". Melanoma Management. 1 (2): 151–164. doi:10.2217/mmt.14.23. PMC 6094595. PMID 30190820.
- ^ a b Brunner G, Reitz M, Heinecke A, Lippold A, Berking C, Suter L, et al. (February 2013). "A nine-gene signature predicting clinical outcome in cutaneous melanoma". Journal of Cancer Research and Clinical Oncology. 139 (2): 249–258. doi:10.1007/s00432-012-1322-z. PMID 23052696. S2CID 20257709.
- ^ Swanson NA, Lee KK, Gorman A, Lee HN (October 2002). "Biopsy techniques. Diagnosis of melanoma". Dermatologic Clinics. 20 (4): 677–680. doi:10.1016/S0733-8635(02)00025-6. PMID 12380054.
- ^ Thomas D, Bello DM (March 2021). "Adjuvant immunotherapy for melanoma". Journal of Surgical Oncology. 123 (3): 789–797. doi:10.1002/jso.26329. ISSN 0022-4790. PMID 33595889. S2CID 231943836.
- ^ Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH (January 1996). "Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684". Journal of Clinical Oncology. 14 (1): 7–17. doi:10.1200/JCO.1996.14.1.7. PMID 8558223. S2CID 24020903.
- ^ Mocellin S, Lens MB, Pasquali S, Pilati P, Chiarion Sileni V (June 2013). "Interferon alpha for the adjuvant treatment of cutaneous melanoma". The Cochrane Database of Systematic Reviews. 2013 (6): CD008955. doi:10.1002/14651858.CD008955.pub2. PMC 10773707. PMID 23775773. Archived from the original on 12 June 2018. Retrieved 11 June 2018.
- ^ Wheatley K, Ives N, Eggermont A, Kirkwood J, Cascinelli N, Markovic SN, et al. (2007). "Interferon-α as an adjuvant therapy for melanoma: an individual patient meta-analysis of randomised trials". J Clin Oncol. 25 (18 Suppl): 8526. doi:10.1200/jco.2007.25.18_suppl.8526.
- ^ Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, et al. (June 2000). "High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190". Journal of Clinical Oncology. 18 (12): 2444–2458. doi:10.1200/JCO.2000.18.12.2444. PMID 10856105.
- ^ Kirkwood JM, Ibrahim JG, Sondak VK, Ernstoff MS, Ross M (March 2002). "Interferon alfa-2a for melanoma metastases". Lancet. 359 (9310): 978–979. doi:10.1016/S0140-6736(02)08001-7. PMID 11918944. S2CID 33866115.
- ^ a b c d e f g h i j k l m Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S (February 2018). "Systemic treatments for metastatic cutaneous melanoma". The Cochrane Database of Systematic Reviews. 2 (2): CD011123. doi:10.1002/14651858.CD011123.pub2. PMC 6491081. PMID 29405038.
- ^ Ryden V, El-Naggar AI, Koliadi A, Ladjevardi CO, Digkas E, Valachis A, et al. (29 December 2023). "The role of dacarbazine and temozolomide therapy after treatment with immune checkpoint inhibitors in malignant melanoma patients: A case series and meta-analysis". Pigment Cell & Melanoma Research. 37 (3): 352–362. doi:10.1111/pcmr.13156. ISSN 1755-1471. PMID 38158376.
- ^ Garbe C, Terheyden P, Keilholz U, Kölbl O, Hauschild A (5 December 2008). "Treatment of Melanoma". Deutsches Ärzteblatt International. 105 (49): 845–851. doi:10.3238/arztebl.2008.0845. ISSN 1866-0452. PMC 2689638. PMID 19561811.
- ^ O'Donoghue C, Perez MC, Mullinax JE, Hardman D, Sileno S, Naqvi SM, et al. (December 2017). "Isolated Limb Infusion: A Single-Center Experience with Over 200 Infusions". Annals of Surgical Oncology. 24 (13): 3842–3849. doi:10.1245/s10434-017-6107-9. PMC 7771340. PMID 29019175. S2CID 28907006.
- ^ Giles MH, Coventry BJ (August 2013). "Isolated limb infusion chemotherapy for melanoma: an overview of early experience at the Adelaide Melanoma Unit". Cancer Management and Research. 5: 243–249. doi:10.2147/cmar.s45746. PMC 3753062. PMID 23990731.
- ^ Berger M, Richtig G, Kashofer K, Aigelsreiter A, Richtig E (June 2018). "The window of opportunities for targeted therapy in BRAFwt/NRASwt/KITwt melanoma: biology and clinical implications of fusion proteins and other mutations". Giornale Italiano di Dermatologia e Venereologia. 153 (3): 349–360. doi:10.23736/S0392-0488.18.05970-9. PMID 29600692.
- ^ Broussard L, Howland A, Ryu S, Song K, Norris D, Armstrong CA, et al. (September 2018). "Melanoma Cell Death Mechanisms". Chonnam Medical Journal. 54 (3): 135–142. doi:10.4068/cmj.2018.54.3.135. PMC 6165917. PMID 30288368.
- ^ a b c d Maverakis E, Cornelius LA, Bowen GM, Phan T, Patel FB, Fitzmaurice S, et al. (May 2015). "Metastatic melanoma - a review of current and future treatment options". Acta Dermato-Venereologica. 95 (5): 516–524. doi:10.2340/00015555-2035. PMID 25520039.
- ^ Kuske M, Westphal D, Wehner R, Schmitz M, Beissert S, Praetorius C, et al. (October 2018). "Immunomodulatory effects of BRAF and MEK inhibitors: Implications for Melanoma therapy". Pharmacological Research. 136: 151–159. doi:10.1016/j.phrs.2018.08.019. PMID 30145328. S2CID 207369519.
- ^ a b c Rebecca VW, Herlyn M (2020). "Nongenetic Mechanisms of Drug Resistance in Melanoma". Annual Review of Cancer Biology. 4: 315–330. doi:10.1146/annurev-cancerbio-030419-033533.
- ^ "FDA Approves First Cellular Therapy to Treat Patients with Unresectable or Metastatic Melanoma". U.S. Food and Drug Administration (Press release). 16 February 2024. Retrieved 18 February 2024.
- ^ "Iovance's Amtagvi (lifileucel) Receives U.S. FDA Accelerated Approval for Advanced Melanoma" (Press release). Iovance Biotherapeutics Inc. 16 February 2024. Retrieved 18 February 2024 – via GlobeNewswire.
- ^ a b c Sanlorenzo M, Vujic I, Posch C, Dajee A, Yen A, Kim S, et al. (June 2014). "Melanoma immunotherapy". Cancer Biology & Therapy. 15 (6): 665–674. doi:10.4161/cbt.28555. PMC 4049781. PMID 24651672.
- ^ Dranitsaris G, Zhu X, Adunlin G, Vincent MD (August 2018). "Cost effectiveness vs. affordability in the age of immuno-oncology cancer drugs". Expert Review of Pharmacoeconomics & Outcomes Research. 18 (4): 351–357. doi:10.1080/14737167.2018.1467270. PMID 29681201. S2CID 5079628.
- ^ a b c d West HJ (April 2015). "JAMA Oncology Patient Page. Immune Checkpoint Inhibitors". JAMA Oncology. 1 (1): 115. doi:10.1001/jamaoncol.2015.0137. PMID 26182315.
- ^ "Interleukin-2 (IL2) for Metastatic Melanoma". Melanoma Research Alliance. Retrieved 5 October 2022.
- ^ Buzaid AC (October 2004). "Management of metastatic cutaneous melanoma". Oncology. 18 (11): 1443–50, discussion 1457–9. PMID 15609471.
- ^ Rosenberg SA, Restifo NP (April 2015). "Adoptive cell transfer as personalized immunotherapy for human cancer". Science. 348 (6230): 62–68. Bibcode:2015Sci...348...62R. doi:10.1126/science.aaa4967. PMC 6295668. PMID 25838374.
- ^ Zang YW, Gu XD, Xiang JB, Chen ZY (June 2014). "Clinical application of adoptive T cell therapy in solid tumors". Medical Science Monitor. 20: 953–959. doi:10.12659/msm.890496. PMC 4063985. PMID 24912947.
- ^ "U.S. Food and Drug Administration Approves First LAG-3-Blocking Antibody Combination, Opdualag (nivolumab and relatlimab-rmbw), as Treatment for Patients with Unresectable or Metastatic Melanoma" (Press release). Bristol Myers Squibb. 18 March 2022. Archived from the original on 19 March 2022. Retrieved 19 March 2022 – via Business Wire.
- ^ a b Tzellos T, Kyrgidis A, Mocellin S, Chan AW, Pilati P, Apalla Z (December 2014). "Interventions for melanoma in situ, including lentigo maligna". The Cochrane Database of Systematic Reviews. 2016 (12): CD010308. doi:10.1002/14651858.CD010308.pub2. PMC 11005944. PMID 25526608.
- ^ Johnson TM, Headington JT, Baker SR, Lowe L (November 1997). "Usefulness of the staged excision for lentigo maligna and lentigo maligna melanoma: the "square" procedure". Journal of the American Academy of Dermatology. 37 (5 Pt 1): 758–764. doi:10.1016/S0190-9622(97)70114-2. PMID 9366823.
- ^ Hill R, Healy B, Holloway L, Kuncic Z, Thwaites D, Baldock C (March 2014). "Advances in kilovoltage x-ray beam dosimetry". Physics in Medicine and Biology. 59 (6): R183–R231. Bibcode:2014PMB....59R.183H. doi:10.1088/0031-9155/59/6/r183. PMID 24584183. S2CID 18082594.
- ^ Bastiaannet E, Beukema JC, Hoekstra HJ (February 2005). "Radiation therapy following lymph node dissection in melanoma patients: treatment, outcome and complications". Cancer Treatment Reviews. 31 (1): 18–26. doi:10.1016/j.ctrv.2004.09.005. PMID 15707701.
- ^ Ford MB, Mitchell MF, Boyer KL, Ford MB, Judkins AF, Levin B (1999). "Cancer Epidemiology". Primary Care Oncology. pp. 1–27.
- ^ Homsi J, Kashani-Sabet M, Messina JL, Daud A (October 2005). "Cutaneous melanoma: prognostic factors". Cancer Control. 12 (4): 223–229. doi:10.1177/107327480501200403. PMID 16258493.
- ^ Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. (August 2001). "Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma". Journal of Clinical Oncology. 19 (16): 3635–3648. CiteSeerX 10.1.1.475.6560. doi:10.1200/JCO.2001.19.16.3635. PMID 11504745. Archived from the original on 5 March 2006. Retrieved 31 July 2006.
- ^ Bae JM, Choi YY, Kim DS, Lee JH, Jang HS, Lee JH, et al. (January 2015). "Metastatic melanomas of unknown primary show better prognosis than those of known primary: a systematic review and meta-analysis of observational studies". Journal of the American Academy of Dermatology. 72 (1): 59–70. doi:10.1016/j.jaad.2014.09.029. PMID 25440435.
- ^ Zhan H, Ma JY, Jian QC (September 2018). "Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in melanoma patients: A meta-analysis". Clinica Chimica Acta; International Journal of Clinical Chemistry. 484: 136–140. doi:10.1016/j.cca.2018.05.055. PMID 29856976. S2CID 44155588.
- ^ Wade RG, Robinson AV, Lo MC, Keeble C, Marples M, Dewar DJ, et al. (October 2018). "Baseline Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios as Biomarkers of Survival in Cutaneous Melanoma: A Multicenter Cohort Study". Annals of Surgical Oncology. 25 (11): 3341–3349. doi:10.1245/s10434-018-6660-x. PMC 6132419. PMID 30066226.
- ^ Robinson AV, Keeble C, Lo MC, Thornton O, Peach H, Moncrieff MD, et al. (April 2020). "The neutrophil-lymphocyte ratio and locoregional melanoma: a multicentre cohort study". Cancer Immunology, Immunotherapy. 69 (4): 559–568. doi:10.1007/s00262-019-02478-7. PMC 7113207. PMID 31974724.
- ^ "CANCERMondial (GLOBOCAN)". GLOBOCAN. 2010. Archived from the original on 17 February 2012. Retrieved 12 August 2010.
- ^ Berwick M, Wiggins C (May 2006). "The current epidemiology of cutaneous malignant melanoma". Frontiers in Bioscience. 11: 1244–1254. doi:10.2741/1877. PMID 16368510.
- ^ "Cancer in Australia: an overview 2012". AIHW. Archived from the original on 2 June 2014. Retrieved 1 June 2014.
- ^ U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2020 submission data (1999-2018): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, released in June 2021.
- ^ a b Guy GP, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC (June 2015). "Vital signs: melanoma incidence and mortality trends and projections - United States, 1982-2030". MMWR. Morbidity and Mortality Weekly Report. 64 (21): 591–596. PMC 4584771. PMID 26042651. Archived from the original on 31 May 2017.
- ^ "CDC – Skin Cancer Statistics". www.cdc.gov. Archived from the original on 10 April 2017. Retrieved 10 April 2017.
- ^ "Key Statistics for Melanoma Skin Cancer". www.cancer.org. Archived from the original on 10 April 2017. Retrieved 10 April 2017.
- ^ Urteaga O, Pack GT (May 1966). "On the antiquity of melanoma". Cancer. 19 (5): 607–610. doi:10.1002/1097-0142(196605)19:5<607::AID-CNCR2820190502>3.0.CO;2-8. PMID 5326247.
- ^ Bodenham DC (October 1968). "A study of 650 observed malignant melanomas in the South-West region". Annals of the Royal College of Surgeons of England. 43 (4): 218–239. PMC 2312310. PMID 5698493.
- ^ Laennec RT (1806). "Sur les melanoses". Bulletin de la Faculté de Médecine de Paris. 1: 24–26.
- ^ Norris W (October 1820). "Case of Fungoid Disease". Edinburgh Medical and Surgical Journal. 16 (65): 562–565. PMC 5830209. PMID 30332089.
- ^ Norris W. Eight cases of Melanosis with pathological and therapeutical remarks on that disease. London: Longman; 1857.
- ^ Cooper S (1844). The First Lines of the Theory and Practice of Surgery: Including the Principle Operations. S.S. and W. Wood. Archived from the original on 4 August 2021. Retrieved 14 December 2017.
- ^ Merriam-Webster, Merriam-Webster's Collegiate Dictionary, Merriam-Webster, archived from the original on 10 October 2020, retrieved 20 July 2018
- ^ Liddell HG, Scott R. "μέλας". A Greek-English Lexicon. Perseus. Archived from the original on 5 June 2011.
- ^ "Dorland's Illustrated Medical Dictionary". Elsevier. Archived from the original on 11 January 2014. Retrieved 20 July 2018.
- ^ "The American Heritage Dictionary of the English Language". Houghton Mifflin Harcourt. Archived from the original on 25 September 2015. Retrieved 20 July 2018.
- ^ "Drugs in Clinical Development for Melanoma". Pharmaceutical Medicine. 26 (3): 171–83. 23 December 2012. doi:10.1007/BF03262391. S2CID 13247048. Archived from the original on 1 January 2013. Retrieved 12 June 2012.
- ^ Sotomayor MG, Yu H, Antonia S, Sotomayor EM, Pardoll DM (2002). "Advances in gene therapy for malignant melanoma". Cancer Control. 9 (1): 39–48. doi:10.1177/107327480200900106. PMID 11907465.
- ^ Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. (November 2008). "Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens". Journal of Clinical Oncology. 26 (32): 5233–5239. doi:10.1200/JCO.2008.16.5449. PMC 2652090. PMID 18809613.
- ^ Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. (May 2010). "Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients" (PDF). Clinical Cancer Research. 16 (9): 2646–2655. doi:10.1158/1078-0432.CCR-10-0041. PMID 20406835. S2CID 32568. Retrieved 15 August 2011.
- ^ "New Method of Gene Therapy Alters Immune Cells for Treatment of Advanced Melanoma; Technique May Also Apply to Other Common Cancers". 30 December 2015. Archived from the original on 28 September 2006.
- ^ "Immune System Taught To Fight Melanoma". CBSNews. 30 May 2009. Archived from the original on 29 October 2010.
- ^ Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. (June 2011). "gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma". The New England Journal of Medicine. 364 (22): 2119–2127. doi:10.1056/NEJMoa1012863. PMC 3517182. PMID 21631324.
- ^ Harmon A (21 February 2010). "A Roller Coaster Chase for a Cure". The New York Times. Archived from the original on 10 February 2017.
- ^ a b Pollack A (5 June 2011). "Drugs Show Promise Slowing Advanced Melanoma". The New York Times. Archived from the original on 31 January 2017.
- ^ Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. (June 2011). "Improved survival with vemurafenib in melanoma with BRAF V600E mutation". The New England Journal of Medicine. 364 (26): 2507–2516. doi:10.1056/NEJMoa1103782. PMC 3549296. PMID 21639808.
- ^ "GSK melanoma drugs add to tally of U.S. drug approvals". Reuters. 30 May 2013. Archived from the original on 24 September 2015.
- ^ "Combination of dabrafenib and trametinib delays development of treatment resistance in MM patients". News Medical. 1 October 2012. Archived from the original on 14 May 2013.
- ^ Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. (November 2012). "Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations". The New England Journal of Medicine. 367 (18): 1694–1703. doi:10.1056/NEJMoa1210093. PMC 3549295. PMID 23020132.
- ^ "Dabrafenib/Trametinib Combination Approved for Advanced Melanoma". OncLive. 9 January 2014. Archived from the original on 25 January 2014. Retrieved 4 February 2015.
- ^ Shirley M (August 2018). "Encorafenib and Binimetinib: First Global Approvals". Drugs. 78 (12): 1277–1284. doi:10.1007/s40265-018-0963-x. PMID 30117021. S2CID 52020890.
- ^ "Counteracting Drug Resistance in Melanoma". 2015. Archived from the original on 4 February 2015.
- ^ "Bristol drug cuts death risk in advanced melanoma". Reuters. 5 June 2010. Archived from the original on 9 November 2010.
- ^ "The Risk For Bristol". Forbes. Archived from the original on 15 March 2011.
- ^ "Phase 3 clinical study: Ipilimumab boosts, sustains immune system responses against melanoma tumors". News-medical.net. 9 June 2010. Archived from the original on 19 October 2012. Retrieved 13 August 2012.
- ^ Jefferson E (25 March 2011). "FDA approves new treatment for a type of late-stage skin cancer" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on 27 March 2011. Retrieved 25 March 2011.
- ^ Pollack A (25 March 2011). "Approval for Drug That Treats Melanoma". The New York Times. Archived from the original on 1 April 2011. Retrieved 27 March 2011.
- ^ "Yervoy". Drugs.com. Archived from the original on 9 August 2011.
- ^ Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. (June 2011). "Ipilimumab plus dacarbazine for previously untreated metastatic melanoma". The New England Journal of Medicine. 364 (26): 2517–2526. doi:10.1056/NEJMoa1104621. PMID 21639810.
- ^ Voit C, Van Akkooi AC, Schäfer-Hesterberg G, Schoengen A, Kowalczyk K, Roewert JC, et al. (February 2010). "Ultrasound morphology criteria predict metastatic disease of the sentinel nodes in patients with melanoma". Journal of Clinical Oncology. 28 (5): 847–852. doi:10.1200/JCO.2009.25.7428. PMID 20065175.
- ^ "malignant-melanoma.org". Archived from the original on 14 October 2011.
- ^ Forbes NE, Abdelbary H, Lupien M, Bell JC, Diallo JS (September 2013). "Exploiting tumor epigenetics to improve oncolytic virotherapy". Frontiers in Genetics. 4: 184. doi:10.3389/fgene.2013.00184. PMC 3778850. PMID 24062768.
- ^ Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. (September 2015). "Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma". Journal of Clinical Oncology. 33 (25): 2780–2788. doi:10.1200/JCO.2014.58.3377. PMID 26014293. S2CID 23663167.
- ^ Costa B, Vale N (September 2023). "Exploring HERV-K (HML-2) Influence in Cancer and Prospects for Therapeutic Interventions". International Journal of Molecular Sciences. 24 (19): 1615. doi:10.3390/ijms25031615. PMC 10572383. PMID 37834078.
- ^ Müller MD, Holst PJ, Nielsen KN (January 2022). "A Systematic Review of Expression and Immunogenicity of Human Endogenous Retroviral Proteins in Cancer and Discussion of Therapeutic Approaches". International Journal of Molecular Sciences. 23 (3): 1330. doi:10.3390/ijms23031330. PMC 8836156. PMID 35163254.