Grignard reaction
| Classical Grignard reaction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Named after | Victor Grignard | ||||||||||
| Reaction type | Coupling reaction | ||||||||||
| Reaction | |||||||||||
| |||||||||||
| Identifiers | |||||||||||
| Organic Chemistry Portal | grignard-reaction | ||||||||||
| RSC ontology ID | RXNO:0000014 | ||||||||||

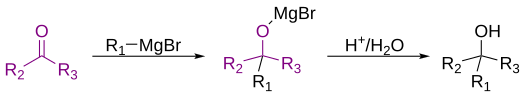
The Grignard reaction (French: [ɡʁiɲaʁ]) is an organometallic chemical reaction in which, according to the classical definition, carbon alkyl, allyl, vinyl, or aryl magnesium halides (Grignard reagent) are added to the carbonyl groups of either an aldehyde or ketone under anhydrous conditions.[1][2][3] This reaction is important for the formation of carbon–carbon bonds.[4][5]
History and definitions
[edit]Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard (University of Nancy, France), who described them in 1900.[6] He was awarded the 1912 Nobel Prize in Chemistry for this work.[7]
The reaction of an organic halide with magnesium is not a Grignard reaction, but provides a Grignard reagent.[8]

Classically, the Grignard reaction refers to the reaction between a ketone or aldehyde group with a Grignard reagent to form a primary or tertiary alcohol.[1] However, some chemists understand the definition to mean all reactions of any electrophiles with Grignard reagents.[9] Therefore, there is some dispute about the modern definition of the Grignard reaction. In the Merck Index, published online by the Royal Society of Chemistry, the classical definition is acknowledged, followed by "A more modern interpretation extends the scope of the reaction to include the addition of Grignard reagents to a wide variety of electrophilic substrates."[9] This variety of definitions illustrates that there is some dispute within the chemistry community about the definition of a Grignard reaction.
Shown below are some reactions involving Grignard reagents, but they themselves are not classically understood as Grignard reactions.

Reaction mechanism
[edit]Because carbon is more electronegative than magnesium, the carbon attached to magnesium acts as a nucleophile and attacks the electrophilic carbon atom in the polar bond of a carbonyl group. The addition of the Grignard reagent to the carbonyl group typically proceeds through a six-membered ring transition state, as shown below.[10]

Based on the detection of radical coupling side products, an alternative single electron transfer (SET) mechanism that involves the initial formation of a ketyl radical intermediate has also been proposed.[11] A recent computational study suggests that the operative mechanism (polar vs. radical) is substrate-dependent, with the reduction potential of the carbonyl compound serving as a key parameter.[12]
Conditions
[edit]
The Grignard reaction is conducted under anhydrous conditions.[3] Otherwise, the reaction will fail because the Grignard reagent will act as a base rather than a nucleophile and pick up a labile proton rather than attacking the electrophilic site. This will result in no formation of the desired product as the R-group of the Grignard reagent will become protonated while the MgX portion will stabilize the deprotonated species.
To prevent this, Grignard reactions are completed in an inert atmosphere to remove all water from the reaction flask and ensure that the desired product is formed.[13] Additionally, if there are acidic protons in the starting material, as shown in the figure on the right, one can overcome this by protecting the acidic site of the reactant by turning it into an ether or a silyl ether to eliminate the labile proton from the solution prior to the Grignard reaction.
Variants
[edit]Other variations of the Grignard reagent have been discovered to improve the chemoselectivity of the Grignard reaction, which include but are not limited to: Turbo-Grignards, organocerium reagents, and organocuprate (Gilman) reagents.
Turbo-Grignards
[edit]Turbo-Grignards are Grignard reagents modified with lithium chloride. Compared to conventional Grignard reagents, Turbo-Grignards are more chemoselective; esters, amides, and nitriles do not react with the Turbo-Grignard reagent.[14]

Heterometal-modified Grignard reagents
[edit]
The behavior of Grignard reagents can be usefully modified in the present of other metals. Copper(I) salts give organocuprates that preferentially effect 1,4 addition.[15] Cerium trichloride allows selective 1,2-additions to the same substrates. Nickel and palladium halides catalyze cross coupling reactions.
See also
[edit]- Grignard reagent
- Wittig reaction
- Horner–Wadsworth–Emmons reaction
- Barbier reaction
- Bodroux–Chichibabin aldehyde synthesis
- Fujimoto–Belleau reaction
- Organolithium reagents
- Sakurai reaction
- Indium-mediated allylation
- Alkynylation
References
[edit]- ^ a b Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Chapter 19: Carboxylic Acids. Organic Chemistry 4e Carey. mhhe.com
- ^ a b c Ouellette, Robert J.; Rawn, J. David (January 1, 2014), Ouellette, Robert J.; Rawn, J. David (eds.), "15 - Alcohols: Reactions and Synthesis", Organic Chemistry, Boston: Elsevier, pp. 491–534, doi:10.1016/b978-0-12-800780-8.00015-2, ISBN 978-0-12-800780-8, retrieved November 6, 2023
- ^ Shirley, D. A. (1954). "The Synthesis of Ketones from Acid Halides and Organometallic Compounds of Magnesium, Zinc, and Cadmium". Org. React. 8: 28–58.
- ^ Huryn, D. M. (1991). "Carbanions of Alkali and Alkaline Earth Cations: (ii) Selectivity of Carbonyl Addition Reactions". In Trost, B. M.; Fleming, I. (eds.). Comprehensive Organic Synthesis, Volume 1: Additions to C—X π-Bonds, Part 1. Elsevier Science. pp. 49–75. doi:10.1016/B978-0-08-052349-1.00002-0. ISBN 978-0-08-052349-1.
- ^ texte, Académie des sciences (France) Auteur du (January 1, 1900). "Comptes rendus hebdomadaires des séances de l'Académie des sciences / publiés... par MM. les secrétaires perpétuels". Gallica. Retrieved April 23, 2023.
- ^ Grignard, V. (1900). "Sur quelques nouvelles combinaisons organométaliques du magnésium et leur application à des synthèses d'alcools et d'hydrocabures". Compt. Rend. 130: 1322–25.
- ^ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). ISBN 0-9678550-9-8. doi:10.1351/goldbook.
- ^ a b "Grignard Reaction | The Merck Index Online". www.rsc.org. Retrieved April 23, 2023.
- ^ Maruyama, K.; Katagiri, T. (1989). "Mechanism of the Grignard reaction". J. Phys. Org. Chem. 2 (3): 205–213. doi:10.1002/poc.610020303.
- ^ Ashby, E. C.; Goel, A. B. (August 1981). "Direct evidence supporting a single electron transfer pathway in the reduction of ketones by primary, secondary, and tertiary Grignard reagents". Journal of the American Chemical Society. 103 (16): 4983–4985. doi:10.1021/ja00406a070. ISSN 0002-7863.
- ^ Peltzer, Raphael Mathias; Gauss, Jürgen; Eisenstein, Odile; Cascella, Michele (February 12, 2020). "The Grignard Reaction – Unraveling a Chemical Puzzle". Journal of the American Chemical Society. 142 (6): 2984–2994. doi:10.1021/jacs.9b11829. hdl:10852/83918. ISSN 0002-7863. PMID 31951398. S2CID 210709021.
- ^ Carey, Francis A. "Grignard reagent". Britannica.
- ^ Hermann, Andreas; Seymen, Rana; Brieger, Lukas; Kleinheider, Johannes; Grabe, Bastian; Hiller, Wolf; Strohmann, Carsten (2023). "Comprehensive Study of the Enhanced Reactivity of Turbo-Grignard Reagents*". Angewandte Chemie International Edition. 62 (25). doi:10.1002/anie.202302489. PMID 36971042. S2CID 257765567.
- ^ Woodward, Simon (January 1, 2000). "Decoding the 'black box' reactivity that is organocuprate conjugate addition chemistry". Chemical Society Reviews. 29 (6): 393–401. doi:10.1039/B002690P. ISSN 1460-4744.
