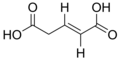
Glutaconic acid
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Pent-2-enedioic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H6O4 | |||
| Molar mass | 130.099 g/mol | ||
| Appearance | Colorless solid | ||
| Melting point | 137 to 139 °C (279 to 282 °F; 410 to 412 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
trans-Glutaconic acid is an organic compound with formula HO2CCH=CHCH2CO2H. This dicarboxylic acid exists as a colorless solid and is related to the saturated chemical glutaric acid, HO2CC(CH2)3CO2H. Esters and salts of glutaconic acid are called glutaconates.
Glutaconate bound to coenzyme A, glutaconyl-CoA, is an intermediate in lysine metabolism.[1]
Related compounds
[edit]The geometric isomer, cis-glutaconic acid, has a noticeably lower melting point (130–132 °C). It can be prepared by bromination of levulinic acid followed by treatment of the dibromoketone with potassium carbonate.[2]
Glutaconic anhydride, which forms by dehydration the diacid, exists mainly as the dicarbonyl tautomer in solution. It is a colorless solid melting at 77–82 °C. Either the cis or trans diacid can be used to make it: the trans form isomerizes under the reaction conditions.[3]
Medical aspects
[edit]Glutaric, 3-hydroxyglutaric, and glutaconic acids are structurally related metabolites. In glutaric aciduria type 1, glutaconic acid accumulates, resulting in brain damage.
References
[edit]- ^ Voet, Donald; Voet, Judith G. (2011). Biochemistry (4 ed.). Hoboken, NJ: Wiley. pp. 1040–1041. ISBN 978-0-470-57095-1.
- ^ Buckel, W.; Pierik, A. J.; Plett, S.; Alhapel, A.; Suarez, D.; Tu, S.-m.; Golding, B. T. (2006). "Mechanism-Based Inactivation of Coenzyme B12-Dependent 2-Methyleneglutarate Mutase by (Z)-Glutaconate and Buta-1,3-diene-2,3-dicarboxylate". Eur. J. Inorg. Chem. 2006 (18): 3622–3626. doi:10.1002/ejic.200600405.
- ^ Briggs, S. P.; Davies, D. I.; Newton, R. F.; Reynolds, D. P. (1981). "The Structure of Glutaconic Anhydride and the Synthetic Utility of its Diels–Alder Adduct with Cyclopentadiene". J. Chem. Soc. Perkin Trans. 1. 146: 146–149. doi:10.1039/P19810000146.