Osazone
Appearance
(Redirected from Glucosazone)

Osazone are a class of carbohydrate derivatives found in organic chemistry formed when reducing sugars are reacted with excess of phenylhydrazine at boiling temperatures.[1][2]
Formation
[edit]Osazone formation was developed by Emil Fischer,[3] who used the reaction as a test to identify monosaccharides.
The formation of a pair of hydrazone functionalities involves both oxidation and condensation reactions.[4] Since the reaction requires a free carbonyl group, only "reducing sugars" participate. Sucrose, which is nonreducing, does not form an osazone.
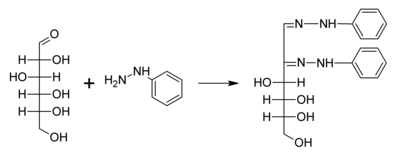
A typical reaction showing the formation of an osazone. D-glucose reacts with phenylhydrazine to give glucosazone. The same product is obtained from fructose and mannose.
Appearance
[edit]Osazones are highly coloured and crystalline compounds. Osazones are readily distinguished.[5]
- Maltosazone (from maltose) forms petal-shaped crystals.
- Lactosazone (from lactose) forms powder puff-shaped crystals.
- Galactosazone (from galactose) forms rhombic-plate shaped crystals.
- Glucosazone (from glucose, fructose or mannose) forms broomstick or needle-shaped crystals.
Historic references
[edit]- Fischer, Emil (1908). "Schmelzpunkt des Phenylhydrazins und einiger Osazone". Berichte der Deutschen Chemischen Gesellschaft. 41: 73–77. doi:10.1002/cber.19080410120.
- Fischer, Emil (1894). "Ueber einige Osazone und Hydrazone der Zuckergruppe". Berichte der Deutschen Chemischen Gesellschaft. 27 (2): 2486–2492. doi:10.1002/cber.189402702249.
- Barry, VINCENT C.; Mitchell, PW (1955). "Mechanism of Osazone Formation". Nature. 175 (4448): 220. Bibcode:1955Natur.175..220B. doi:10.1038/175220a0. PMID 13235861.
References
[edit]- ^ El Khadem, Hassan S.; Fatiadi, Alexander J. (2000). "Hydrazine derivatives of carbohydrates and related compounds". Advances in Carbohydrate Chemistry and Biochemistry. 55: 175-263. doi:10.1016/S0065-2318(00)55006-9. ISBN 9780120072552. Retrieved 31 March 2021.
- ^ Mester, L.; El Khadem, H.; Horton, D. (1970). "Structure of saccharide osazones". Journal of the Chemical Society C: Organic (18): 2567. doi:10.1039/J39700002567.
- ^ Helferich, B. (1953). "Emil Fischer zum 100. Geburtstag". Angewandte Chemie. 65 (2): 45–52. Bibcode:1953AngCh..65...45H. doi:10.1002/ange.19530650202.
- ^ Ramakrishnan, S. (2004). Textbook of Medical Biochemistry. Orient Blackswan. ISBN 9788125020714.
- ^ Gupta, Anil (2019). "Carbohydrates". Comprehensive Biochemistry for Dentistry. Singapore: Springer. pp. 108–110. ISBN 978-981-13-1035-5.


