Encapsulin
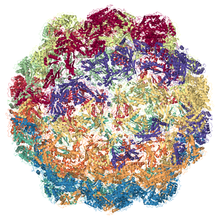 EM structure of Myxococcus xanthus encpasulin protein (EncA) PDB entry 4pt2 | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Linocin_M18 | ||||||||
| Pfam | PF04454 | ||||||||
| InterPro | IPR007544 | ||||||||
| |||||||||
The encapsulins are a family of bacterial proteins that serve as the main structural components of encapsulin nanocompartments.[1] There are several different encapsulin proteins, including EncA, which forms the shell, and EncB, EncC, and EncD, which form the core.[1]
Encapsulins are also used in synthetic biology. They are hard to discover due to their similarity to phage proteins.[2]
History
[edit]Encapsulins were discovered in 1994 as a new class of prokaryotic compartments.[3] Prokaryotic cells usually lack membrane compartments typical for eukaryotes. They instead have numerous protein compartments that are capable of accumulating a large number of molecules.[3]
When protein nanocompartments were discovered in 1994, and later renamed encapsulins, they were found in the supernatant fluid of the Brevibacterium linens culture.[3]
In 2008, encapsulins were recognized as protein-based compartmentalization systems with dedicated functions in cellular organisms.[4]
Structure
[edit]Encapsulin shells compromise icosahedral complexes (12 vertices, 20 faces, 30 edges) formed as a result of self-assembly of protomers. [3] The shells have diameters between 24 and 42 nm and are defined by the viral HK97-fold of their shell protein. [4] The HK97-fold protomer is roughly triangular and has three conserved domains, the axial domain, peripheral domain, and extended loop.[4]
Function
[edit]Encapsulins serve many physiological functions, including catalysis, mineral storage, response to oxidative stress and secondary metabolism. There are ferritin-like encapsulins as well.[2]
References
[edit]- ^ a b McHugh CA, Fontana J, Nemecek D, Cheng N, Aksyuk AA, Heymann JB, Winkler DC, Lam AS, Wall JS, Steven AC, Hoiczyk E (September 2014). "A virus capsid-like nanocompartment that stores iron and protects bacteria from oxidative stress". The EMBO Journal. 33 (17): 1896–911. doi:10.15252/embj.201488566. PMC 4195785. PMID 25024436.
- ^ a b Kashif-Khan N, Savva R, Frank S (7 March 2024). "Mining metagenomics data for novel bacterial nanocompartments". NAR Genomics & Bioinformatics. 6 (1): lqae025. doi:10.1093/nargab/lqae025.
- ^ a b c d Chmelyuk, Nelly S.; Oda, Vera V.; Gabashvili, Anna N.; Abakumov, Maxim A. (17 Feb 2023). "Encapsulins: Structure, Properties, and Biotechnological Applications". Biochemistry (Moscow). 88 (1): 35–49. doi:10.1134/S0006297923010042. ISSN 0006-2979. Retrieved 17 Oct 2024 – via National Library of Medicine.
- ^ a b c Giessen, Tobias W. (18 Mar 2022). "Encapsulins". Annual Review of Biochemistry. 91 (1): 353–380. doi:10.1146/annurev-biochem-040320-102858. ISSN 0066-4154. PMC 9944552. PMID 35303791.
