Thiamine deficiency
| Thiamine deficiency[1] | |
|---|---|
| Other names | Beriberi, vitamin B1 deficiency, thiamine-deficiency syndrome[1][2] |
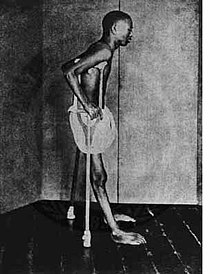 | |
| Sufferer of beriberi in Southeast Asia beginning of the 20th Century | |
| Specialty | Neurology, cardiology, pediatrics |
| Symptoms | |
| Types | Wet, dry, gastrointestinal,[3] infantile,[4] cerebral[5] |
| Causes | Not enough thiamine[1] |
| Risk factors | Diet of mostly white rice; alcoholism, dialysis, chronic diarrhea, diuretics[1][6] |
| Prevention | Food fortification, Food diversification[1] |
| Treatment | Thiamine supplementation[1] |
| Frequency | Uncommon (USA)[1] |
Thiamine deficiency is a medical condition of low levels of thiamine (vitamin B1).[1] A severe and chronic form is known as beriberi.[1][7] The name beriberi was possibly borrowed in the 18th century from the Sinhalese phrase බැරි බැරි (bæri bæri, “I cannot, I cannot”), owing to the weakness caused by the condition. The two main types in adults are wet beriberi and dry beriberi.[1] Wet beriberi affects the cardiovascular system, resulting in a fast heart rate, shortness of breath, and leg swelling.[1] Dry beriberi affects the nervous system, resulting in numbness of the hands and feet, confusion, trouble moving the legs, and pain.[1] A form with loss of appetite and constipation may also occur.[3] Another type, acute beriberi, found mostly in babies, presents with loss of appetite, vomiting, lactic acidosis, changes in heart rate, and enlargement of the heart.[8]
Risk factors include a diet of mostly white rice, alcoholism, dialysis, chronic diarrhea, and taking high doses of diuretics.[1][6] In rare cases, it may be due to a genetic condition that results in difficulties absorbing thiamine found in food.[1] Wernicke encephalopathy and Korsakoff syndrome are forms of dry beriberi.[6] Diagnosis is based on symptoms, low levels of thiamine in the urine, high blood lactate, and improvement with thiamine supplementation.[9]
Treatment is by thiamine supplementation, either by mouth or by injection.[1] With treatment, symptoms generally resolve in a few weeks.[9] The disease may be prevented at the population level through the fortification of food.[1]
Thiamine deficiency is rare in the United States.[10] It remains relatively common in sub-Saharan Africa.[2] Outbreaks have been seen in refugee camps.[6] Thiamine deficiency has been described for thousands of years in Asia, and became more common in the late 1800s with the increased processing of rice.[11]
Signs and symptoms
[edit]Symptoms of beriberi include weight loss, emotional disturbances, impaired sensory perception, weakness and pain in the limbs, and periods of irregular heart rate. Edema (swelling of bodily tissues) is common. It may increase the amount of lactic acid and pyruvic acid within the blood. In advanced cases, the disease may cause high-output cardiac failure and death.
Symptoms may occur concurrently with those of Wernicke's encephalopathy, a primarily neurological thiamine deficiency-related condition.
Beriberi is divided into four categories. The first three are historical and the fourth, gastrointestinal beriberi, was recognized in 2004:
- Dry beriberi especially affects the peripheral nervous system.
- Wet beriberi especially affects the cardiovascular system and other bodily systems.
- Infantile beriberi affects the babies of malnourished mothers.
- Gastrointestinal beriberi affects the digestive system and other bodily systems.
Dry beriberi
[edit]Dry beriberi causes wasting and partial paralysis resulting from damaged peripheral nerves. It is also referred to as endemic neuritis. It is characterized by:
- Difficulty with walking
- Tingling or loss of sensation (numbness) in hands and feet
- Loss of tendon reflexes[12]
- Loss of muscle function or paralysis of the lower legs
- Mental confusion/speech difficulties
- Pain
- Involuntary eye movements (nystagmus)
- Vomiting
A selective impairment of the large proprioceptive sensory fibers without motor impairment can occur and present as a prominent sensory ataxia, which is a loss of balance and coordination due to loss of the proprioceptive inputs from the periphery and loss of position sense.[13]
Brain disease
[edit]Wernicke's encephalopathy (WE), Korsakoff syndrome (also called alcohol amnestic disorder), and Wernicke–Korsakoff syndrome are forms of dry beriberi.[6]
Wernicke's encephalopathy is the most frequently encountered manifestation of thiamine deficiency in Western society,[14][15] though it may also occur in patients with impaired nutrition from other causes, such as gastrointestinal disease,[14] those with HIV/AIDS, and with the injudicious administration of parenteral glucose or hyperalimentation without adequate B-vitamin supplementation.[16] This is a striking neuro-psychiatric disorder characterized by paralysis of eye movements, abnormal stance and gait, and markedly deranged mental function.[17]
Korsakoff syndrome, in general, is considered to occur with deterioration of brain function in patients initially diagnosed with WE.[18] This is an amnestic-confabulatory syndrome characterized by retrograde and anterograde amnesia, impairment of conceptual functions, and decreased spontaneity and initiative.[19]
Alcoholics may have thiamine deficiency because of:
- Inadequate nutritional intake: Alcoholics tend to intake less than the recommended amount of thiamine.
- Decreased uptake of thiamine from the GI tract: Active transport of thiamine into enterocytes is disturbed during acute alcohol exposure.
- Liver thiamine stores are reduced due to hepatic steatosis or fibrosis.[20]
- Impaired thiamine utilization: Magnesium, which is required for the binding of thiamine to thiamine-using enzymes within the cell, is also deficient due to chronic alcohol consumption. The inefficient use of any thiamine that does reach the cells will further exacerbate the thiamine deficiency.
- Ethanol per se inhibits thiamine transport in the gastrointestinal system and blocks phosphorylation of thiamine to its cofactor form (ThDP).[21]
Following improved nutrition and the removal of alcohol consumption, some impairments linked with thiamine deficiency are reversed, in particular poor brain functionality, although in more severe cases, Wernicke–Korsakoff syndrome leaves permanent damage. (See delirium tremens.)
Wet beriberi
[edit]Wet beriberi affects the heart and circulatory system. It is sometimes fatal, as it causes a combination of heart failure and weakening of the capillary walls, which causes the peripheral tissues to become edematous. Wet beriberi is characterized by:
- Increased heart rate
- Vasodilation leading to decreased systemic vascular resistance, and high-output heart failure[22]
- Elevated jugular venous pressure[23]
- Dyspnea (shortness of breath) on exertion
- Paroxysmal nocturnal dyspnea
- Peripheral edema[23] (swelling of lower legs) or generalized edema[24][25][26][27] (swelling throughout the body)
- Dilated cardiomyopathy
Gastrointestinal beriberi
[edit]Gastrointestinal beriberi causes abdominal pain. It is characterized by:
Infants
[edit]Infantile beriberi usually occurs between two and six months of age in children whose mothers have inadequate thiamine intake. It may present as either wet or dry beriberi.[2]
In the acute form, the baby develops dyspnea and cyanosis and soon dies of heart failure. These symptoms may be described in infantile beriberi:
- Hoarseness, where the child makes moves to moan, but emits no sound or just faint moans[30] caused by nerve paralysis[12]
- Weight loss, becoming thinner and then marasmic as the disease progresses[30]
- Vomiting[30]
- Diarrhea[30]
- Pale skin[12]
- Edema[12][30]
- Ill temper[12]
- Alterations of the cardiovascular system, especially tachycardia (rapid heart rate)[12]
- Convulsions occasionally observed in the terminal stages[30]
Cause
[edit]Beriberi is often caused by eating a diet with a very high proportion of calorie rich polished rice (common in Asia) or cassava root (common in sub-Saharan Africa), without much if any thiamine-containing animal products or vegetables.[2]
It may also be caused by shortcomings other than inadequate intake – diseases or operations on the digestive tract, alcoholism,[23] dialysis or genetic deficiencies. All those causes mainly affect the central nervous system, and provoke the development of Wernicke's encephalopathy.
Wernicke's disease is one of the most prevalent neurological or neuropsychiatric diseases.[31] In autopsy series, features of Wernicke lesions are observed in approximately 2% of general cases.[32] Medical record research shows that about 85% had not been diagnosed, although only 19% would be asymptomatic. In children, only 58% were diagnosed. In alcohol abusers, autopsy series showed neurological damages at rates of 12.5% or more. Mortality caused by Wernicke's disease reaches 17% of diseases, which means 3.4/1000 or about 25 million contemporaries.[33][34] The number of people with Wernicke's disease may be even higher, considering that early stages may have dysfunctions prior to the production of observable lesions at necropsy. In addition, uncounted numbers of people can experience fetal damage and subsequent diseases.
Genetics
[edit]Genetic diseases of thiamine transport are rare but serious. Thiamine responsive megaloblastic anemia syndrome (TRMA) with diabetes mellitus and sensorineural deafness[35] is an autosomal recessive disorder caused by mutations in the gene SLC19A2,[36] a high affinity thiamine transporter. TRMA patients do not show signs of systemic thiamine deficiency, suggesting redundancy in the thiamine transport system. This has led to the discovery of a second high-affinity thiamine transporter, SLC19A3.[37][38] Leigh disease (subacute necrotising encephalomyelopathy) is an inherited disorder that affects mostly infants in the first years of life and is invariably fatal. Pathological similarities between Leigh disease and WE led to the hypothesis that the cause was a defect in thiamine metabolism. One of the most consistent findings has been an abnormality of the activation of the pyruvate dehydrogenase complex.[39]
Mutations in the SLC19A3 gene have been linked to biotin-thiamine responsive basal ganglia disease,[40] which is treated with pharmacological doses of thiamine and biotin, another B vitamin.
Other disorders in which a putative role for thiamine has been implicated include subacute necrotising encephalomyelopathy, opsoclonus myoclonus syndrome (a paraneoplastic syndrome), and Nigerian seasonal ataxia (or African seasonal ataxia). In addition, several inherited disorders of ThDP-dependent enzymes have been reported,[41] which may respond to thiamine treatment.[19]
Pathophysiology
[edit]Thiamine in the human body has a half-life of 17 days and is quickly exhausted, particularly when metabolic demands exceed intake. A derivative of thiamine, thiamine pyrophosphate (TPP), is a cofactor involved in the citric acid cycle, as well as connecting the breakdown of sugars with the citric acid cycle. The citric acid cycle is a central metabolic pathway involved in the regulation of carbohydrate, lipid, and amino acid metabolism, and its disruption due to thiamine deficiency inhibits the production of many molecules including the neurotransmitters glutamic acid and GABA.[42] Additionally, thiamine may also be directly involved in neuromodulation.[43]
Diagnosis
[edit]
A positive diagnosis test for thiamine deficiency involves measuring the activity of the enzyme transketolase in erythrocytes (Erythrocyte transketolase activation assay). Alternatively, thiamine and its phosphorylated derivatives can directly be detected in whole blood, tissues, foods, animal feed, and pharmaceutical preparations following the conversion of thiamine to fluorescent thiochrome derivatives (thiochrome assay) and separation by high-performance liquid chromatography (HPLC).[44][45][46] Capillary electrophoresis (CE) techniques and in-capillary enzyme reaction methods have emerged as alternative techniques in quantifying and monitoring thiamine levels in samples.[47] The normal thiamine concentration in EDTA-blood is about 20–100 μg/L.
Treatment
[edit]Many people with beriberi can be treated with thiamine alone.[48] Given thiamine intravenously (and later orally), rapid and dramatic[23] recovery occurs, generally within 24 hours.[49]
Improvements of peripheral neuropathy may require several months of thiamine treatment.[50]
Epidemiology
[edit]Beriberi is a recurrent nutritional disease in detention houses, even in this century. In 1999, an outbreak of beriberi occurred in a detention center in Taiwan.[51] High rates of illness and death from beriberi in overcrowded Haitian jails in 2007 were traced to the traditional practice of washing rice before cooking; this removed a nutritious coating which had been applied to the rice after processing (enriched white rice).[52] In the Ivory Coast, among a group of prisoners with heavy punishment, 64% were affected by beriberi. Before beginning treatment, prisoners exhibited symptoms of dry or wet beriberi with neurological signs (tingling: 41%), cardiovascular signs (dyspnoea: 42%, thoracic pain: 35%), and edemas of the lower limbs (51%). With treatment, the rate of healing was about 97%.[53]
Populations under extreme stress may be at higher risk for beriberi. Displaced populations, such as refugees from war, are susceptible to micronutritional deficiency, including beriberi.[54] The severe nutritional deprivation caused by famine also can cause beriberis, although symptoms may be overlooked in clinical assessment or masked by other famine-related problems.[55] An extreme weight-loss diet can, rarely, induce a famine-like state and the accompanying beriberi.[23]
Workers on Chinese squid ships are at elevated risk of beriberi due to the simple carbohydrate-rich diet they are fed and the long period of time between shoring. Between 2013 and 2021, 15 workers on 14 ships have died with symptoms of beriberi.[56]
History
[edit]Earliest written descriptions of thiamine deficiency are from ancient China in the context of Chinese medicine. One of the earliest is by Ge Hong in his book Zhou hou bei ji fang (Emergency Formulas to Keep up Your Sleeve) written sometime during the third century. Hong called the illness by the name jiao qi, which can be interpreted as "foot qi". He described the symptoms to include swelling, weakness, and numbness of the feet. He also acknowledged that the illness could be deadly, and claimed that it could be cured by eating certain foods, such as fermented soybeans in wine. Better known examples of early descriptions of "foot qi" are by Chao Yuanfang (who lived during 550–630) in his book Zhu bing yuan hou lun (Sources and Symptoms of All Diseases)[57][58] and by Sun Simiao (581–682) in his book Bei ji qian jin yao fang (Essential Emergency Formulas Worth a Thousand in Gold).[59][58][60][61]
In the mid-19th century, interest in beriberi steadily rose as the disease became more noticeable with changes in diet in East and Southeast Asia. There was a steady uptick in medical publications, reaching one hundred and eighty-one publications from 1880 and 1889, and hundreds more in the following decades. The link to white rice was clear to Western doctors, but a confounding factor was that some other foods like meat failed to prevent beriberi, so it could not be easily explained as a lack of known chemicals like carbon or nitrogen. With no knowledge of vitamins, the etiology of beriberi was among the most hotly debated subjects in Victorian medicine.[62]
The first successful preventative measure against beriberi was discovered by Takaki Kanehiro, a British-trained Japanese medical doctor of the Imperial Japanese Navy, in the mid-1880s.[63] Beriberi was a serious problem in the Japanese navy; sailors fell ill an average of four times a year in the period 1878 to 1881, and 35% were cases of beriberi.[63] In 1882, Takaki learned of a very high incidence of beriberi among cadets on a training mission from Japan to Hawaii, via New Zealand and South America. The voyage lasted more than nine months and resulted in 169 cases of sickness and 25 deaths on a ship of 376 men. Takaki observed that beriberi was common among low-ranking crew who were often provided free rice, thus ate little else, but not among crews of Western navies, nor among Japanese officers who consumed a more varied diet. With the support of the Japanese Navy, he conducted an experiment in which another ship was deployed on the same route, except that its crew was fed a diet of meat, fish, barley, rice, and beans. At the end of the voyage, this crew had only 14 cases of beriberi and no deaths.[63] This emphasis on varied diet contradicted observations by other doctors, and Takaki's carbon-based etiology was just as incorrect as similar theories before him, but the results of his experiment impressed the Japanese Navy, which adopted his proposed solution. By 1887 beriberi had been completely eliminated on Navy ships.[62]
In the same year, Takaki's experiment was described favorably in The Lancet,[64] but his incorrect etiology was not taken seriously.[65] In 1897, Christiaan Eijkman, a Dutch physician and pathologist, published his mid-1880s experiments showing that feeding unpolished rice (instead of the polished variety) to chickens helped to prevent beriberi.[66] This was the first experiment to show that not a major chemical but some minor nutrient was the true cause of beriberi. The following year, Sir Frederick Hopkins postulated that some foods contained "accessory factors"—in addition to proteins, carbohydrates, fats, and salt—that were necessary for the functions of the human body.[67][68] In 1901, Gerrit Grijns, a Dutch physician and assistant to Christiaan Eijkman in the Netherlands, correctly interpreted beriberi as a deficiency syndrome,[69] and between 1910 and 1913, Edward Bright Vedder established that an extract of rice bran is a treatment for beriberi.[citation needed] In 1929, Eijkman and Hopkins were awarded the Nobel Prize for Physiology or Medicine for their discoveries.
Japanese Army denialism
[edit]Although the identification of beriberi as a deficiency syndrome was proven beyond a doubt by 1913, a Japanese group headed by Mori Ōgai and backed by Tokyo Imperial University continued to deny this conclusion until 1926. In 1886, Mori, then working in the Japanese Army Medical Bureau, asserted that white rice was sufficient as a diet for soldiers. Simultaneously, Navy surgeon general Takaki Kanehiro published the groundbreaking results described above. Mori, who had been educated under German doctors, responded that Takaki was a "fake doctor" due to his lack of prestigious medical background, while Mori himself and his fellow graduates of Tokyo Imperial University constituted the only "real doctors" in Japan and that they alone were capable of "experimental induction", although Mori himself had not conducted any beriberi experiments.[70]
The Japanese Navy sided with Takaki and adopted his suggestions. In order to prevent himself and the Army from losing face, Mori assembled a team of doctors and professors from Tokyo Imperial University and the Japanese Army who proposed that beriberi was caused by an unknown pathogen, which they described as etowasu (from the German Etwas, meaning "something"). They employed various social tactics to denounce vitamin deficiency experiments and prevent them from being published, while beriberi ravaged the Japanese Army. During the First Sino-Japanese War and Russo-Japanese War, Army soldiers continued to die in mass numbers from beriberi, while Navy sailors survived. In response to this severe loss of life, in 1907, the Army ordered the formation of a Beriberi Emergency Research Council, headed by Mori. Its members pledged to find the cause of beriberi.[71] By 1919, with most Western doctors acknowledging that beriberi was a deficiency syndrome, the Emergency Research Council began conducting experiments using various vitamins, but stressed that "more research was necessary". During this period, more than 300,000 Japanese soldiers contracted beriberi and over 27,000 died.[72]
Mori died in 1922. The Beriberi Research Council disbanded in 1925, and by the time Eijkman and Hopkins were awarded the Nobel Prize, all of its members had acknowledged that beriberi was a deficiency syndrome.
Etymology
[edit]Although according to the Oxford English Dictionary, the term "beriberi" comes from a Sinhalese phrase meaning "weak, weak" or "I cannot, I cannot", the word being duplicated for emphasis,[73] the origin of the phrase is questionable. It has also been suggested to come from Hindi, Arabic, and a few other languages, with many meanings like "weakness", "sailor", and even "sheep". Such suggested origins were listed by Heinrich Botho Scheube, among others. Edward Vedder wrote in his book Beriberi (1913) that "it is impossible to definitely trace the origin of the word beriberi". The word berbere was used in writing at least as early as 1568 by Diogo do Couto, when he described the deficiency in India.[74]
Kakke (脚気), which is a Japanese synonym for thiamine deficiency, comes from the way "jiao qi" is pronounced in Japanese.[75] "Jiao qi" is an old word used in Chinese medicine to describe beriberi.[57] "Kakke" is supposed to have entered into the Japanese language sometime between the sixth and eighth centuries.[75]
Other animals
[edit]Poultry
[edit]Mature chickens show signs three weeks after being fed a deficient diet. In young chicks, it can appear before two weeks of age. Onset is sudden in young chicks, with anorexia and an unsteady gait. Later on, locomotor signs begin, with an apparent paralysis of the flexor of the toes. The characteristic position is called "stargazing", with the affected animal sitting on its hocks with its head thrown back in a posture called opisthotonos. Response to administration of the vitamin is rather quick, occurring a few hours later.[76][77]
Ruminants
[edit]Polioencephalomalacia (PEM) is the most common thiamine deficiency disorder in young ruminant and nonruminant animals. Symptoms of PEM include a profuse, but transient, diarrhea, listlessness, circling movements, stargazing or opisthotonus (head drawn back over neck), and muscle tremors.[78] The most common cause is high-carbohydrate feeds, leading to the overgrowth of thiaminase-producing bacteria, but dietary ingestion of thiaminase (e.g., in bracken fern), or inhibition of thiamine absorption by high sulfur intake are also possible.[79] Another cause of PEM is Clostridium sporogenes or Bacillus aneurinolyticus infection. These bacteria produce thiaminases that can cause an acute thiamine deficiency in the affected animal.[80]
Snakes
[edit]Snakes that consume a diet largely composed of goldfish and feeder minnows are susceptible to developing thiamine deficiency. This is often a problem observed in captivity when keeping garter and ribbon snakes that are fed a goldfish-exclusive diet, as these fish contain thiaminase, an enzyme that breaks down thiamine.[81]
Wild birds and fish
[edit]Thiamine deficiency has been identified as the cause of a paralytic disease affecting wild birds in the Baltic Sea area dating back to 1982.[82] In this condition, there is difficulty in keeping the wings folded along the side of the body when resting, loss of the ability to fly and voice, with eventual paralysis of the wings and legs and death. It affects primarily 0.5–1 kg-sized birds such as the European herring gull (Larus argentatus), common starling (Sturnus vulgaris), and common eider (Somateria mollissima). Researchers noted, "Because the investigated species occupy a wide range of ecological niches and positions in the food web, we are open to the possibility that other animal classes may develop thiamine deficiency, as well."[82]p. 12006
In the counties of Blekinge and Skåne, mass deaths of several bird species, especially the European herring gull, have been observed since the early 2000s. More recently, species of other classes seems to be affected. High mortality of salmon (Salmo salar) in the river Mörrumsån is reported, and mammals such as the Eurasian elk (Alces alces) have died in unusually high numbers. Lack of thiamine is the common denominator where analysis is done. In April 2012, the County Administrative Board of Blekinge found the situation so alarming that they asked the Swedish government to set up a closer investigation.[83]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r "Beriberi". Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. 2015. Archived from the original on 11 November 2017. Retrieved 11 November 2017.
- ^ a b c d Adamolekun B, Hiffler L (24 October 2017). "A diagnosis and treatment gap for thiamine deficiency disorders in sub-Saharan Africa?". Annals of the New York Academy of Sciences. 1408 (1): 15–19. Bibcode:2017NYASA1408...15A. doi:10.1111/nyas.13509. PMID 29064578.
- ^ a b Ferri FF (2017). Ferri's Clinical Advisor 2018 E-Book: 5 Books in 1. Elsevier Health Sciences. p. 1368. ISBN 978-0-323-52957-0. Archived from the original on 2017-11-11.
- ^ "Pediatric Beriberi Clinical Presentation: History, Physical Examination". emedicine.medscape.com. Retrieved 2024-04-10.
- ^ Arányi J (1991-11-24). "[Cerebral beriberi with ophthalmoplegia as the leading symptom in an alcoholic patient]". Orvosi Hetilap. 132 (47): 2627–2628. ISSN 0030-6002. PMID 1956687.
- ^ a b c d e "Nutrition and Growth Guidelines". Domestic Guidelines - Immigrant and Refugee Health. CDC. March 2012. Archived from the original on 11 November 2017. Retrieved 11 November 2017.
- ^ Hermann W, Obeid R (2011). Vitamins in the prevention of human diseases. Berlin: Walter de Gruyter. p. 58. ISBN 978-3-11-021448-2.
- ^ Gropper SS, Smith JL (2013). Advanced Nutrition and Human Metabolism (6 ed.). Wadsworth, Cengage Learning. p. 324. ISBN 978-1-133-10405-6.
- ^ a b Swaiman KF, Ashwal S, Ferriero DM, Schor NF, Finkel RS, Gropman AL, Pearl PL, Shevell M (2017). Swaiman's Pediatric Neurology E-Book: Principles and Practice. Elsevier Health Sciences. p. e929. ISBN 978-0-323-37481-1. Archived from the original on 2017-11-11.
- ^ "Thiamine Fact Sheet for Consumers". Office of Dietary Supplements (ODS): USA.gov. Archived from the original on October 29, 2017. Retrieved April 10, 2018.
- ^ Lanska DJ (2010). "Chapter 30 Historical aspects of the major neurological vitamin deficiency disorders: The water-soluble B vitamins". History of Neurology. Handbook of Clinical Neurology. Vol. 95. pp. 445–76. doi:10.1016/S0072-9752(08)02130-1. ISBN 978-0-444-52009-8. PMID 19892133.
- ^ a b c d e f Katsura E, Oiso T (1976). Beaton G, Bengoa J (eds.). "Chapter 9. Beriberi" (PDF). World Health Organization Monograph Series No. 62: Nutrition in Preventive Medicine. Geneva: World Health Organization. Archived from the original (PDF) on 2011-07-08.
- ^ Spinazzi M, Angelini C, Patrini C (2010). "Subacute sensory ataxia and optic neuropathy with thiamine deficiency". Nature Reviews Neurology. 6 (5): 288–93. doi:10.1038/nrneurol.2010.16. PMID 20308997. S2CID 12333200.
- ^ a b Kril JJ (1996). "Neuropathology of thiamine deficiency disorders". Metab Brain Dis. 11 (1): 9–17. doi:10.1007/BF02080928. PMID 8815394. S2CID 20889916.
- ^ For an interesting discussion on thiamine fortification of foods, specifically targetting beer, see "Wernicke's encephalopathy and thiamine fortification of food: time for a new direction?". Medical Journal of Australia. Archived from the original on 2011-08-31.
- ^ Butterworth RF, Gaudreau C, Vincelette J, et al. (1991). "Thiamine deficiency and Wernicke's encephalopathy in AIDS". Metab Brain Dis. 6 (4): 207–12. doi:10.1007/BF00996920. PMID 1812394. S2CID 8833558.
- ^ Harper C (1979). "Wernicke's encephalopathy, a more common disease than realised (a neuropathological study of 51 cases)". J Neurol Neurosurg Psychiatry. 42 (3): 226–231. doi:10.1136/jnnp.42.3.226. PMC 490724. PMID 438830.
- ^ McCollum EV A History of Nutrition. Cambridge, MA: Riverside Press, Houghton Mifflin; 1957.
- ^ a b Butterworth RF. Thiamin. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease, 10th ed. Baltimore: Lippincott Williams & Wilkins; 2006.
- ^ Butterworth RF (1993). "Pathophysiologic mechanisms responsible for the reversible (thiamine-responsive) and irreversible (thiamine non-responsive) neurological symptoms of Wernicke's encephalopathy". Drug Alcohol Rev. 12 (3): 315–22. doi:10.1080/09595239300185371. PMID 16840290.
- ^ Rindi G, Imarisio L, Patrini C (1986). "Effects of acute and chronic ethanol administration on regional thiamin pyrophosphokinase activity of the rat brain". Biochem Pharmacol. 35 (22): 3903–8. doi:10.1016/0006-2952(86)90002-X. PMID 3022743.
- ^ Anand IS, Florea VG (2001). "High Output Cardiac Failure". Current Treatment Options in Cardiovascular Medicine. 3 (2): 151–159. doi:10.1007/s11936-001-0070-1. PMID 11242561. S2CID 44475541.
- ^ a b c d e McIntyre N, Stanley NN (1971). "Cardiac Beriberi: Two Modes of Presentation". BMJ. 3 (5774): 567–9. doi:10.1136/bmj.3.5774.567. PMC 1798841. PMID 5571454.
- ^ Lee HS, Lee SA, Shin HS, Choi HM, Kim SJ, Kim HK, Park YB (August 2013). "A case of cardiac beriberi: a forgotten but memorable disease". Korean Circulation Journal. 43 (8): 569–572. doi:10.4070/kcj.2013.43.8.569. ISSN 1738-5520. PMC 3772304. PMID 24044018.
- ^ Tanabe N, Hiraoka E, Kataoka J, Naito T, Matsumoto K, Arai J, Norisue Y (March 2018). "Wet Beriberi Associated with Hikikomori Syndrome". Journal of General Internal Medicine. 33 (3): 384–387. doi:10.1007/s11606-017-4208-6. ISSN 1525-1497. PMC 5834955. PMID 29188542.
- ^ Watson JT, El Bushra H, Lebo EJ, Bwire G, Kiyengo J, Emukule G, Omballa V, Tole J, Zuberi M, Breiman RF, Katz MA (2011). "Outbreak of beriberi among African Union troops in Mogadishu, Somalia". PLOS ONE. 6 (12): e28345. Bibcode:2011PLoSO...628345W. doi:10.1371/journal.pone.0028345. ISSN 1932-6203. PMC 3244391. PMID 22205947.
- ^ Toyonaga J, Masutani K, Tsuruya K, Haruyama N, Sugiwaka S, Suehiro T, Maeda H, Taniguchi M, Katafuchi R, Iida M (October 2009). "Severe anasarca due to beriberi heart disease and diabetic nephropathy". Clinical and Experimental Nephrology. 13 (5): 518–521. doi:10.1007/s10157-009-0189-z. ISSN 1437-7799. PMID 19459028. S2CID 38559672.
- ^ Donnino M (2004). "Gastrointestinal Beriberi: A Previously Unrecognized Syndrome". Ann Intern Med. 141 (11): 898–899. doi:10.7326/0003-4819-141-11-200412070-00035. PMID 15583247.
- ^ Duca, J., Lum, C., & Lo, A. (2015). Elevated Lactate Secondary to Gastrointestinal Beriberi. J GEN INTERN MED Journal of General Internal Medicine
- ^ a b c d e f Latham MC (1997). "Chapter 16. Beriberi and thiamine deficiency". Human nutrition in the developing world (Food and Nutrition Series – No. 29). Fao Food and Nutrition Series. Rome: Food and Agriculture Organization of the United Nations (FAO). ISSN 1014-3181. Archived from the original on 2014-02-03.
- ^ Cernicchiaro L (2007). "Enfermedad de Wernicke (o Encefalopatía de Wernicke). Monitoring an acute and recovered case for twelve years" [Wernicke´s Disease (or Wernicke´s Encephalopathy)] (in Spanish). Archived from the original on 2013-05-22.
- ^ Salen PN (1 March 2013). Kulkarni R (ed.). "Wernicke Encephalopathy". Medscape. Archived from the original on 12 May 2013.
- ^ Harper CG, Giles M, Finlay-Jones R (April 1986). "Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy". J Neurol Neurosurg Psychiatry. 49 (4): 341–5. doi:10.1136/jnnp.49.4.341. PMC 1028756. PMID 3701343.
- ^ Harper C (March 1979). "Wernicke's encephalopathy: a more common disease than realised. A neuropathological study of 51 cases". J Neurol Neurosurg Psychiatry. 42 (3): 226–31. doi:10.1136/jnnp.42.3.226. PMC 490724. PMID 438830.
- ^ Slater PV (1978). "Thiamine Responsive Megaloblastic Anemia with severe diabetes mellitus and sensorineural deafness (TRMA)". The Australian Nurses' Journal. 7 (11): 40–3. PMID 249270.
- ^ Kopriva V, Bilkovic, R, Licko, T (Dec 1977). "Tumours of the small intestine (author's transl)". Ceskoslovenska Gastroenterologie a Vyziva. 31 (8): 549–53. ISSN 0009-0565. PMID 603941.
- ^ Beissel J (Dec 1977). "The role of right catheterization in valvular prosthesis surveillance (author's transl)". Annales de Cardiologie et d'Angéiologie. 26 (6): 587–9. ISSN 0003-3928. PMID 606152.
- ^ Online Mendelian Inheritance in Man (OMIM): 249270
- ^ Butterworth RF. Pyruvate dehydrogenase deficiency disorders. In: McCandless DW, ed. Cerebral Energy Metabolism and Metabolic Encephalopathy. Plenum Publishing Corp.; 1985.
- ^ Tabarki B, Al-Hashem A, Alfadhel M (July 28, 1993). "Biotin-Thiamine-Responsive Basal Ganglia Disease". In Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Mirzaa G, Amemiya A (eds.). GeneReviews®. University of Washington, Seattle. PMID 24260777. Archived from the original on May 9, 2018 – via PubMed.
- ^ Blass JP. Inborn errors of pyruvate metabolism. In: Stanbury JB, Wyngaarden JB, Frederckson DS et al., eds. Metabolic Basis of Inherited Disease. 5th ed. New York: McGraw-Hill, 1983.
- ^ Sechi G, Serra A (May 2007). "Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management". Lancet Neurology. 6 (5): 442–55. doi:10.1016/S1474-4422(07)70104-7. PMID 17434099. S2CID 15523083.
- ^ Hirsch JA, Parrott J (2012). "New considerations on the neuromodulatory role of thiamine". Pharmacology. 89 (1–2): 111–6. doi:10.1159/000336339. PMID 22398704. S2CID 22555167.
- ^ Bettendorff L, Peeters M, Jouan C, Wins P, Schoffeniels E (1991). "Determination of thiamin and its phosphate esters in cultured neurons and astrocytes using an ion-pair reversed-phase high-performance liquid chromatographic method". Anal. Biochem. 198 (1): 52–59. doi:10.1016/0003-2697(91)90505-N. PMID 1789432.
- ^ Losa R, Sierra MI, Fernández A, Blanco D, Buesa J (2005). "Determination of thiamine and its phosphorylated forms in human plasma, erythrocytes and urine by HPLC and fluorescence detection: a preliminary study on cancer patients". J Pharm Biomed Anal. 37 (5): 1025–1029. doi:10.1016/j.jpba.2004.08.038. PMID 15862682.
- ^ Lu J, Frank E (May 2008). "Rapid HPLC measurement of thiamine and its phosphate esters in whole blood". Clin. Chem. 54 (5): 901–906. doi:10.1373/clinchem.2007.099077. PMID 18356241.
- ^ Shabangi M, Sutton J (2005). "Separation of thiamin and its phosphate esters by capillary zone electrophoresis and its application to the analysis of water-soluble vitamins". Journal of Pharmaceutical and Biomedical Analysis. 38 (1): 66–71. doi:10.1016/j.jpba.2004.11.061. PMID 15907621.
- ^ Dieu-Thu Nguyen-Khoa, Ginette V Busschots, Phyllis A Vallee (2022-06-29), Romesh Khardori (ed.), "Beriberi (Thiamine Deficiency) Treatment & Management: Approach Considerations, Activity", Medscape, archived from the original on 2014-03-24
- ^ Tanphaichitr V. Thiamin. In: Shils ME, Olsen JA, Shike M et al., editors. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Lippincott Williams & Wilkins; 1999
- ^ Maurice V, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd ed. Philadelphia: FA Davis, 1989.
- ^ Chen KT, Twu SJ, Chiou ST, Pan WH, Chang HJ, Serdula MK (2003). "Outbreak of beriberi among illegal mainland Chinese immigrants at a detention center in Taiwan". Public Health Rep. 118 (1): 59–64. doi:10.1093/phr/118.1.59. PMC 1497506. PMID 12604765.
- ^ Sprague J, Alexandra E (17 January 2007). "Haiti: Mysterious Prison Ailment Traced to U.S. Rice". Inter Press Service. Archived from the original on 30 May 2013.
- ^ Aké-Tano O, Konan EY, Tetchi EO, Ekou FK, Ekra D, Coulibaly A, Dagnan NS (2011). "Le béribéri, maladie nutritionnelle récurrente en milieu carcéral en Côte-d'Ivoire". Bulletin de la Société de Pathologie Exotique. 104 (5): 347–351. doi:10.1007/s13149-011-0136-6. PMID 21336653. S2CID 116433417.
- ^ Prinzo ZW, de Benoist B (2009). "Meeting the challenges of micronutrient deficiencies in emergency-affected populations" (PDF). Proceedings of the Nutrition Society. 61 (2): 251–7. doi:10.1079/PNS2002151. PMID 12133207. S2CID 8286320. Archived (PDF) from the original on 2012-10-19.
- ^ Golden M (May 1997). "Diagnosing Beriberi in Emergency Situations". Field Exchange (1): 18. Archived from the original on 2012-10-19.
- ^ Ian Urbina, Joe Galvin, Maya Martin, Susan Ryan, Daniel Murphy, Austin Brush (7 November 2023). "They catch squid for the world's table. But deckhands on Chinese ships pay a deadly price". Los Angeles Times.
- ^ a b HA Smith, p. 26-28
- ^ a b Benedict CA (October 2018). "Forgotten Disease: Illnesses Transformed in Chinese Medicine by Hilary A. Smith (review)". Bulletin of the History of Medicine. 92 (3): 550–51. doi:10.1353/bhm.2018.0059. ISSN 1086-3176. S2CID 80778431.
- ^ HA Smith, p. 44
- ^ "TCM history V the Sui & Tang Dynasties". Archived from the original on 2017-07-14. Retrieved 2017-07-11.
- ^ "Sun Simiao: Author of the Earliest Chinese Encyclopedia for Clinical Practice". Archived from the original on 2007-07-04. Retrieved 2007-06-15.
- ^ a b Carter KC (April 1977). "The germ theory, beriberi, and the deficiency theory of disease" (PDF). Medical History. 21 (2): 126–7. doi:10.1017/S0025727300037662. ISSN 2048-8343. PMC 1081945. PMID 325303.
- ^ a b c Itokawa Y (1976). "Kanehiro Takaki (1849–1920): A Biographical Sketch". Journal of Nutrition. 106 (5): 581–8. doi:10.1093/jn/106.5.581. PMID 772183.
- ^ The Lancet. J. Onwhyn. 1887.
- ^ Yamashita N, Aikawa T (1 March 2017). "Dutch Research on Beriberi: I. Christiaan Eijkman's Research and Evaluation of Kanehiro Takaki's Diet Reforms of the Japanese Navy". Nihon ishigaku zasshi [Journal of Japanese history of medicine]. 63 (1): 3–21. ISSN 0549-3323. PMID 30549780.
- ^ Eijkman C (June 1897). "Eine Beri Beri-ähnliche Krankheit der Hühner". Archiv für pathologische Anatomie und Physiologie und für klinische Medicin (in German). 148 (3): 523–32. doi:10.1007/BF01937576. ISSN 1432-2307.
- ^ Challem J (1997). "The Past, Present and Future of Vitamins". Archived from the original on 8 June 2010.[unreliable medical source?]
- ^ "Christiaan Eijkman, Beriberi and Vitamin B1". Nobelprize.org, Nobel Media AB. Archived from the original on 17 January 2010. Retrieved 8 July 2013.
- ^ Grijns G (1901). "Over polyneuritis gallinarum". Geneeskundig Tijdschrift voor Nederlandsch-Indie. 43: 3–110.
- ^ Kim He (2014). Doctors of empire: medical and cultural encounters between imperial Germany and Meiji Japan. Toronto: University of Toronto Press. p. 146. ISBN 9781442644403.
- ^ Padilla RR (April 2023). "Efficacy vs. Ideology: The Use of Food Therapies in Preventing and Treating Beriberi in the Japanese Army in the Meiji Era". East Asian Science, Technology and Society. 17 (2): 201–21. doi:10.1080/18752160.2022.2071191. ISSN 1875-2160. S2CID 251484808.
- ^ Bay A (2011). "Ōgai Mori rintarō to kakkefunsō 鴎外森林太郎と脚気紛争 [Mori Ōgai and the Beriberi Dispute] (review)". East Asian Science, Technology and Society. 5 (4): 573–79. doi:10.1215/18752160-1458784. ISSN 1875-2152. S2CID 222093508.
- ^ Oxford English Dictionary: "Beri-beri ... a Cingalese word, f. beri weakness, the reduplication being intensive ...", page 203, 1937
- ^ HA Smith, p. 118-119
- ^ a b HA Smith, p. 149
- ^ R.E. Austic and M.L. Scott, Nutritional deficiency diseases, in Diseases of poultry, ed. by M. S. Hofstad. Ames, Iowa: Iowa State University Press. ISBN 0-8138-0430-2. p. 50.
- ^ "Thiamine Deficiency". Merck Veterinary Manual. 2008. Retrieved 2023-02-16.
- ^ National Research Council. 1996. Nutrient Requirements of Beef Cattle, Seventh Revised Ed. Washington, D.C.: National Academy Press.
- ^ Michel Lévy, ed. (March 2015). "Overview of Polioencephalomalacia". Merck Veterinary Manual. Archived from the original on 2016-03-03. Retrieved 2023-02-16.
- ^ Polioencephalomacia: Introduction Archived 2010-05-28 at the Wayback Machine, "ACES Publications"
- ^ "Update on Common Nutritional Disorders of Captive Reptiles". Archived from the original on 2017-09-13. Retrieved 2017-09-13.
- ^ a b Balk L, Hägerroth PA, Akerman G, Hanson M, Tjärnlund U, Hansson T, Hallgrimsson GT, Zebühr Y, Broman D, Mörner T, Sundberg H, et al. (2009). "Wild birds of declining European species are dying from a thiamine deficiency syndrome". Proc Natl Acad Sci U S A. 106 (29): 12001–12006. Bibcode:2009PNAS..10612001B. doi:10.1073/pnas.0902903106. PMC 2715476. PMID 19597145.
- ^ Blekinge län L (2013). "2012-04-15 500-1380-13 Förhöjd dödlighet hos fågel, lax og älg" (PDF). Archived (PDF) from the original on 2013-12-02.
Further reading
[edit]- Arnold D (2010). "British India and the beri-beri problem". Medical History. 54 (3): 295–314. doi:10.1017/S0025727300004622. PMC 2889456. PMID 20592882.
- Chisholm H, ed. (1911). . Encyclopædia Britannica. Vol. 03 (11th ed.). Cambridge University Press. pp. 774–775.
- Smith HA (2017). Forgotten Disease: Illnesses Transformed in Chinese Medicine. doi:10.1093/jhmas/jry029. ISBN 978-1-5036-0350-9. OCLC 993877848.
External links
[edit] Media related to Beriberi at Wikimedia Commons
Media related to Beriberi at Wikimedia Commons
