Paucimannosylation
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
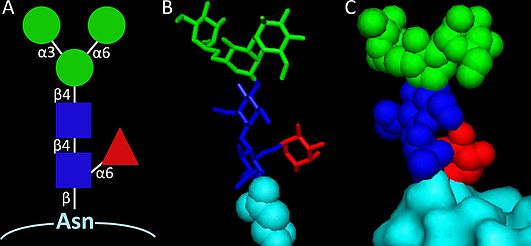
In biochemistry, paucimannosylation is an enzymatic post-translational modification involving the attachment of relatively simple mannose (Man) and N-Acetylglucosamine (GlcNAc) containing carbohydrates (glycans) to proteins.[1] The paucimannosidic glycans may also be modified with other types of monosaccharides including fucose (Fuc) and xylose (Xyl) depending on the species, tissue and cell origin.[2]
Paucimannosylation forms a separate sub-type in the asparagine N-linked glycosylation system. The short paucimannosidic glycans neither structurally nor functionally fit into the three well-established N-glycan classes i.e. oligomannosidic-, hybrid- and complex-type N-glycans.
Paucimannosylation has traditionally been referred to as a N-glycosylation type of "lower organisms",[3] mostly documented in insects, worms and plants. Recent findings have, however, added nuances to this view, by showing their presence and roles in mammals in the areas of immunity, cellular development, pathogen infection and cancer.[4][5] To this end, paucimannosylation is therefore now considered to be a distinct type of N-glycosylation that adds diversity to the highly heterogeneous glycoproteome across the eukaryotic domain.[4][6]
Etymology
[edit]The term "paucimannose" (occasionally spelled as "pauci-mannose") was coined in the early 1990s glycobiology literature.[4] Paucimannose utilises the prefix "pauci" meaning few or small in Latin and the suffix "mannose" indicating glycans involving mannose-terminating glycans.
The phrases protein paucimannosylation and paucimannosidic proteins are commonly used in the literature to describe paucimannose-modified glycoproteins displaying intact structural and functional integrity. In contrast, the oligosaccharides themselves are often referred to as paucimannosidic, low mannose, and truncated glycans or other less conventional nomenclature.[4]
A simple shorthand nomenclature has been proposed as a convenient way to name the individual paucimannosidic glycan structures, e.g. M3F denotes Man3GlcNAc2Fuc1.[6][7][8][9]
Common paucimannosidic structural features across species, tissue and protein origin
[edit]Paucimannosidic glycans span the base composition Man1-3GlcNAc2.[5][10] Additional modifications with Fuc, Xyl and/or Galactose (Gal) are common in mammals ref, plants[4][11] and invertebrates, respectively.[10][12] Paucimannosidic glycans expressed by insects and nematodes are particularly rich in structural diversity.[4]
Tissue expression and (sub-) cellular localisation
[edit]Paucimannosylation has been extensively studied and documented in insects, nematodes and plants over the past decades. The paucimannosidic proteins are constitutively and broadly expressed across tissues in these organisms under normal physiology.[13] It is widely recognised that paucimannosylation is a central component of the glycoproteome in these "lower" organisms.[12] Recently, paucimannosylation was reported to form an unconventional type of protein N-glycosylation in vertebrates.[3] It has been proposed that "higher" species including humans, rodents and other mammals use paucimannosylation in a more tissue- and context-restricted manner in pathophysiological conditions including cancer,[14] pathogen infection, inflammation and stemness.[15]
Insects
[edit]Paucimannosidic glycans form the main component of the N-glycome of insects such as Drosophila melanogaster.[16] Glycoprofiling of the venom component of the western honeybee, Apis mellifera, identified that paucimannosylation is a common modification of key proteins including hyaluronidase and phospholipase.[17][18]
Insect cells lines are frequently utilised for recombinant expression of mammalian glycoproteins, which therefore are decorated with paucimannosidic glycans e.g. mouse interferon-β,[19] human IgG1[20] and calf alkaline phosphatase.[21]
Nematodes
[edit]The model organism Caenorhabditis elegans classified under the phylum Nematoda is amongst the most studied invertebrate species in glycobiology. The literature clearly documents a repertoire of nematodal paucimannosidic glycans.[22] Another model nematode, Pristionchus pacificus, was also documented to express common nematodal paucimannosidic glycans.[10]
Parasitic nematodes such as Haemonchus contortus have been reported to carry paucimannosidic glycans conjugated to an intestinal microsomal aminopeptidase.[23] In addition, there have been reports documenting the expression of paucimannosidic glycans by others parasitic nematodes such as Ascaris suum,[24] Heligmosomoides polygyrus[25] and Trichuris suis.[26]
Plants
[edit]Most plant species studied to date are recognised to constitutively express paucimannosidic N-glycoproteins. The paucimannosidic N-glycoproteins are abundantly expressed in the vacuoles of plants such as the legume seeds of Lotus japonicus,[27] the rice seeds and leaves of Oryza sativa.[28] Literature has provided evidence for plant-specific paucimannosidic glycan structures modified with Xyl and Fuc. Such structures are found across the broad Streptophyta (land plants) and Chlorophyta (green algae) clade and in diatoms such as Phaeodactylum tricornutum.[4] Less reported bixylosylated paucimannosidic glycans have also been documented.[29]
Vertebrates
[edit]Paucimannosidic proteins have been reported in vertebrates such as quail,[30] chicken[31] and in mammals,[6] encompassing a limited diversity of paucimannosidic glycan structures.[4] Early findings reported on paucimannosidic glycans on lysosomal glycoproteins in domestic animals.[32] and human tissues,[33] but have subsequently been found also to decorate non-lysosomal glycoproteins.[34][35] Particularly, the granules of human neutrophils are a principal source of paucimannosidic proteins.[6][7][36][37][38] Paucimannosidic proteins were also observed in human monocytes and macrophages[39] and paucimannosidic immunoglycopeptides were found to be presented by SARS-CoV-2 challenged dendritic cells.[40] Species within other classes under Animalia related to vertebrates were also documented to express paucimannosidic proteins.[41] with some observations of unusual plant- and invertebrate-like paucimannosidic glycan structures[5]
Fungi
[edit]Despite receiving considerable focus, the glycobiological literature do not contain evidence for the presence of paucimannosidic proteins within Fungi. Fungal species within this kingdom are therefore considered devoid of protein paucimannosylation[11] and instead carry high mannosylated N-glycoproteins comprising extended and branched mannose-decorated antennae.[42][43]
Biosynthesis of paucimannosidic N-glycoproteins
[edit]Common aspects of the biosynthesis of paucimannosidic glycoproteins across species
[edit]Similar to other N-linked glycan types, the biosynthesis of paucimannosidic proteins across most species has been documented to be facilitated by the actions of a limited set of glyco-enzymes including beta-N-acetylhexosaminidases (Hex) and alpha-mannosidases, through GnT-I-dependent and -independent truncation pathways.[4]
Insects
[edit]Studies on insect cell lines and in vivo experiments on D. melanogaster have revealed active expression of Hexo1 and Hexo2, and, most importantly, the fused lobe (fdl) gene encoding fused ß-lobe (FDL), also known as GNase, an orthologue of A. thaliana and human Hex. FDL is expressed in high abundance in vesicles and the plasma membrane and has, unlike Hexo1 and Hexo2, been linked to fruit fly paucimannosidic protein production.[44][45][46][47] However, except for the well-studied D. melanogaster and other common insect model organisms, solid evidence for active involvement of Hex and/or the possible concerted usage of the GnT-I-independent pathway or alternative truncation pathways for paucimannosidic protein production remains unavailable across the diverse class of insects.
Nematodes
[edit]The model organism C. elegans is well studied; solid glycobiological literature have provided insights on the nematodal N-glycosylation machinery which shares many traits with other eukaryotic species.[48][49] C. elegans is known to produce paucimannosidic proteins via a GnT-I-dependent route in which GnT-I firstly produces GlcNAc-capped glycoprotein intermediates. Further processing by two Hex isoenzymes (HEX-2 and HEX-3) encoded by two C. elegans genes (hex-2, hex-3) generate the unsubstituted C. elegans paucimannosidic glycans.
Other glycoenzymes catalise further processing and structural diversity including α-Man II and α1,6- and α1,3-fucosyltransferases. Albeit less active, a GnT-I-independent α1,6-fucosyltransferase has also been observed for C. elegans,[50][51] indicating that both the GnT-I-dependent and -independent pathways may contribute to the formation of paucimannosidic N-glycoproteins in worms. However, the biosynthetic processes underpinning the unusual non-sugar and core-modified paucimannosidic N-glycans in C. elegans remain to be elucidated.
Plants
[edit]Hexosaminidases (Hex) are important glycoside hydrolases for the generation of plant-specific paucimannosidic proteins across Plantae. HEXO1-HEXO3 have been reported to be key mediators of paucimannose expression in various plant species including Nicotiana benthamiana,[52] A.thaliana[53] and L. japonicus.[54] Moreover, α1,3-fucosyltransferase (FUT11/12)[55] and β1,2-xylosyltransferase[56] as well as α-mannosidase II[57] were also reported to play critical roles in the generation of the paucimannosidic proteins expressed by plants.[54]
Vertebrates
[edit]In humans, the Hex-mediated GnT-I-dependent truncation pathway is known to facilitate, at least in some tissues including neutrophils, the production of paucimannosidic proteins.[9] Human Hex isoenzymes are assembled with alpha and beta subunits encoded by the HEXA and HEXB genes, respectively.[58] From these two subunits, isoenzymes such as Hex A (one alpha and one beta subunit), Hex B (two beta subunits) and Hex S (two alpha subunits) are generated. Both Hex A and Hex B are reported to play important functional roles in human,[58] particularly in the lysosomal degradation of gangliosides. Recently, both HEXA and HEXB were documented to mediate protein paucimannosylation in human neutrophils[9] and may therefore also be the main driver for the elevated production of paucimannosidic proteins during cancer development.[14] Recent in vitro observations have suggested other noncanonical truncation pathways with direct core fucosylation of paucimannosidic proteins in vertebrates, but this remains to be validated[4] Hex A and Hex B isoenzymes are mainly present in the azurophilic granules of human neutrophils as a result of a proposed targeting-by-timing mechanism that supposedly directs these enzymes to this compartment during neutrophil development.[59] Recently, granule-specific glycosylation was shown in neutrophils featuring prominent paucimannosylation in the azurophilic granules an observation that was suggested to arise from a "glycosylation-by-timing" mechanism yet to be documented.[60] More widely across vertebrate species, the biosynthesis of paucimannosidic proteins remains largely unstudied.
Functions of protein paucimannosylation
[edit]Human
[edit]The function of protein paucimannosylation remains largely unexplored in vertebrates. Recent literature however has emerged demonstrating that paucimannosylation play roles in mediating pathophysiological processes such as in inflammation, pathogen infection, cancer and in the development of stem cells and in normal homeostasis. For example, elevated expression of paucimannosidic proteins was shown in Mycobacterium tuberculosis infected macrophages,[61] during preclampsia[62] and on Tamm-Horsfall proteins secreted by human urothelial cells during urinary tract infections suggesting the involvement of paucimannosylation in those conditions.[63] Additionally, sputum from individuals suffering from cystic fibrosis and airway infections were also observed to be rich in paucimannosidic proteins.[64][65] Furthermore, paucimannosylation was reported to be prominent features of human neutrophils [8][38][66][7] and in monocytes[39] and macrophages.[61] Recent literature have also demonstrated elevated signatures of paucimannosidic proteins associated with a range of human cancers[14] including brain,[67] breast,[68] blood,[61] melanoma,[69] non-melanoma,[70] liver,[71] ovarian[72] and prostate cancers.[73] Enriched paucimannosidic glycoepitopes were found in the tumours when compared to the adjacent non-tumour tissues. Literature have also reported the presence of paucimannosylation in embryonic stem cells[74] and neuronal stem cells,[75] suggesting potential functional role(s) in these cells. Notably, deficiency of hexosaminidases results in clinically significant Tay-Sachs and Sandhoff diseases, which also implicates Hex and paucimannosidic proteins in those conditions.
Endogenous and exogenous binding partners of mammalian paucimannosidic glycans have been suggested,[3] including the macrophage mannose receptor (CD206) and dectin-2. Other putative endogenous paucimannosidic protein receptors such as dectin-1, DC-SIGN and DC-SIGNR have been proposed, but experimental support is still lacking. Exogeneous binders of paucimannosidic glycans such as the Escherichia coli FimH[76] and P. aeruginosa PA-IIL[77] were also reported to play important roles in the adhesion and pathophysiology of these opportunistic pathogens.
Insects
[edit]In D. melanogaster, FDL-deficient mutants showed paucimannose-deficiency and, notably, caused locomotion defects in fruit flies, indicating that Hex and/or paucimannosidic proteins are involved, via elusive pathways, in essential fruit fly processes.[78] As expected, the less-consequential monoallelic fdl mutation was shown to result in reduced paucimannosidic protein formation and caused a non-lethal, but still severe phenotype, by halting the generation of peripheral long-term memory neurons. Impaired generation of peripheral long-term memory neurons[12] was also observed for fruit fly fdl and MgatI null mutations, which, in turn, resulted in infertility and locomotion defects. The lack of fucosylated paucimannosidic glycans was proposed to contribute to neuronal impairment in both fdl and Mgat1 mutants. The importance of fucosylated paucimannosidic glycans was supported by a study reporting that mutations in the FucT6 gene encoding the D. melanogaster α1,6-fucosyltransferase resulted in an impaired fruit fly immune response towards parasitic infections.[79] Taken together, these phenotypic observations suggest that the fruit fly paucimannosidic glycans, some of which overlap with the human repertoire, are pivotal in the development, immune function and survival processes of D. melanogaster. It was reported that T. castaneum abundantly expresses paucimannosidic proteins during its post-larval stages,[80] recapitulating findings from other studies proposing that paucimannosidic proteins are strongly regulated during early development.[15] Thus, it is likely that paucimannosidic glycans conjugated to still unknown flour beetle carrier proteins, similar to those in nematodes and fruit flies, are vital for growth and survival processes of the flour beetle.
Nematodes
[edit]Expression of phosphocholine-modified and unsubstituted C. elegans paucimannosidic glycans is reportedly development stage-specific, implying important roles in nematodal development.[81] In support, C. elegans hex-2 gene knock-out mutants displayed reduced paucimannosidic protein levels and altered sensitivity towards nematotoxic lectins relative to wild-type worms, a correlation suggesting involvement of paucimannosidic proteins in key C. elegans survival processes.[82] Functionally, phosphocholine-containing paucimannosidic glycans were demonstrated to display immune-modulating roles in parasitic nematodes.[83] Paucimannosidic glycans were suggested to play roles in the nematodal innate immune system by impacting the nematode's ability to fight and survive pathogenic bacteria[84]
References
[edit]- ^ a b Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, et al. (December 2015). "Symbol Nomenclature for Graphical Representations of Glycans". Glycobiology. 25 (12): 1323–1324. doi:10.1093/glycob/cwv091. PMC 4643639. PMID 26543186.
- ^ Kubelka, V.; Altmann, F.; Kornfeld, G.; Marz, L. (January 1994). "Structures of the N-Linked Oligosaccharides of the Membrane Glycoproteins from Three Lepidopteran Cell Lines (Sf-21, IZD-Mb-0503, Bm-N)". Archives of Biochemistry and Biophysics. 308 (1): 148–157. doi:10.1006/abbi.1994.1021. ISSN 0003-9861. PMID 8311447.
- ^ a b c Loke, Ian; Kolarich, Daniel; Packer, Nicolle H.; Thaysen-Andersen, Morten (October 2016). "Emerging roles of protein mannosylation in inflammation and infection". Molecular Aspects of Medicine. 51: 31–55. doi:10.1016/j.mam.2016.04.004. ISSN 0098-2997. PMID 27086127.
- ^ a b c d e f g h i j Tjondro, Harry C.; Loke, Ian; Chatterjee, Sayantani; Thaysen-Andersen, Morten (2019-08-14). "Human protein paucimannosylation: cues from the eukaryotic kingdoms". Biological Reviews. 94 (6): 2068–2100. doi:10.1111/brv.12548. ISSN 1464-7931. PMID 31410980. S2CID 199572750.
- ^ a b c Schiller, Birgit; Hykollari, Alba; Yan, Shi; Paschinger, Katharina; Wilson, Iain B.H. (2012-08-01). "Complicated N-linked glycans in simple organisms". BCHM. 393 (8): 661–673. doi:10.1515/hsz-2012-0150. ISSN 1437-4315. PMC 3589692. PMID 22944671.
- ^ a b c d Thaysen-Andersen, Morten; Venkatakrishnan, Vignesh; Loke, Ian; Laurini, Christine; Diestel, Simone; Parker, Benjamin L.; Packer, Nicolle H. (April 2015). "Human Neutrophils Secrete Bioactive Paucimannosidic Proteins from Azurophilic Granules into Pathogen-Infected Sputum". Journal of Biological Chemistry. 290 (14): 8789–8802. doi:10.1074/jbc.m114.631622. ISSN 0021-9258. PMC 4423670. PMID 25645918.
- ^ a b c Loke, Ian; Østergaard, Ole; Heegaard, Niels H.H.; Packer, Nicolle H.; Thaysen-Andersen, Morten (August 2017). "Paucimannose-Rich N-glycosylation of Spatiotemporally Regulated Human Neutrophil Elastase Modulates Its Immune Functions*". Molecular & Cellular Proteomics. 16 (8): 1507–1527. doi:10.1074/mcp.m116.066746. ISSN 1535-9476. PMC 5546201. PMID 28630087. S2CID 19548998.
- ^ a b Ugonotti, Julian; Chatterjee, Sayantani; Thaysen-Andersen, Morten (June 2021). "Structural and functional diversity of neutrophil glycosylation in innate immunity and related disorders". Molecular Aspects of Medicine. 79: 100882. doi:10.1016/j.mam.2020.100882. ISSN 0098-2997. PMID 32847678. S2CID 221345832.
- ^ a b c Ugonotti, Julian; Kawahara, Rebeca; Loke, Ian; Zhu, Yuqi; Chatterjee, Sayantani; Tjondro, Harry C; Sumer-Bayraktar, Zeynep; Neelamegham, Sriram; Thaysen-Andersen, Morten (2021-10-18). "N-acetyl-β-D-hexosaminidases mediate the generation of paucimannosidic proteins via a putative noncanonical truncation pathway in human neutrophils". Glycobiology. 32 (3): 218–229. doi:10.1093/glycob/cwab108. ISSN 1460-2423. PMC 8966476. PMID 34939086.
- ^ a b c Yan, Shi; Wilson, Iain B. H.; Paschinger, Katharina (June 2015). "Comparison of RP-HPLC modes to analyse the N-glycome of the free-living nematodePristionchus pacificus". Electrophoresis. 36 (11–12): 1314–1329. doi:10.1002/elps.201400528. ISSN 0173-0835. PMC 4422755. PMID 25639343.
- ^ a b Wilson, I. B.H.; Zeleny, R.; Kolarich, D.; Staudacher, E.; Stroop, C. J.M.; Kamerling, J. P.; Altmann, F. (2001-04-01). "Analysis of Asn-linked glycans from vegetable foodstuffs: widespread occurrence of Lewis a, core 1,3-linked fucose and xylose substitutions". Glycobiology. 11 (4): 261–274. doi:10.1093/glycob/11.4.261. ISSN 0959-6658. PMID 11358875.
- ^ a b c Schachter, Harry (August 2009). "Paucimannose N-glycans in Caenorhabditis elegans and Drosophila melanogaster". Carbohydrate Research. 344 (12): 1391–1396. doi:10.1016/j.carres.2009.04.028. ISSN 0008-6215. PMID 19515361.
- ^ Essentials of glycobiology. Ajit Varki (2nd ed.). Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press. 2009. ISBN 978-0-87969-770-9. OCLC 195765240.
{{cite book}}: CS1 maint: others (link) - ^ a b c Chatterjee, Sayantani; Lee, Ling Y.; Kawahara, Rebeca; Abrahams, Jodie L.; Adamczyk, Barbara; Anugraham, Merrina; Ashwood, Christopher; Sumer-Bayraktar, Zeynep; Briggs, Matthew T.; Chik, Jenny H. L.; Everest-Dass, Arun (2019-10-16). "Protein Paucimannosylation Is an EnrichedN-Glycosylation Signature of Human Cancers". Proteomics. 19 (21–22): 1900010. doi:10.1002/pmic.201900010. hdl:10072/388125. ISSN 1615-9853. PMID 31419058. S2CID 201018579.
- ^ a b Zipser, Birgit; Diestel, Simone; Bello-DeOcampo, Diana; Tai, Mei-Hui; Schmitz, Brigitte (November 2012). "Mannitou monoclonal antibody uniquely recognizes paucimannose, a marker for human cancer, stemness and inflammation". Journal of Biotechnology. 161: 5. doi:10.1016/j.jbiotec.2012.07.160. ISSN 0168-1656.
- ^ Aoki, Kazuhiro; Perlman, Mindy; Lim, Jae-Min; Cantu, Rebecca; Wells, Lance; Tiemeyer, Michael (March 2007). "Dynamic Developmental Elaboration of N-Linked Glycan Complexity in the Drosophila melanogaster Embryo". Journal of Biological Chemistry. 282 (12): 9127–9142. doi:10.1074/jbc.m606711200. ISSN 0021-9258. PMID 17264077.
- ^ KUBELKA, Viktoria; ALTMANN, Friedrich; STAUDACHER, Erika; TRETTER, Verena; März, Leopold; HARD, Karl; KAMERLING, Johannis P.; VLIEGENTHART, Johannes F. G. (May 1993). "Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2". European Journal of Biochemistry. 213 (3): 1193–1204. doi:10.1111/j.1432-1033.1993.tb17870.x. ISSN 0014-2956. PMID 8504812.
- ^ Kubelka, Viktoria; Altmann, Friedrich; Mrz, Leopold (February 1995). "The asparagine-linked carbohydrate of honeybee venom hyaluronidase". Glycoconjugate Journal. 12 (1): 77–83. doi:10.1007/bf00731872. ISSN 0282-0080. PMID 7795417. S2CID 1543607.
- ^ Misaki, Ryo; Nagaya, Hidekazu; Fujiyama, Kazuhito; Yanagihara, Itaru; Honda, Takeshi; Seki, Tatsuji (November 2003). "N-linked glycan structures of mouse interferon-β produced by Bombyx mori larvae". Biochemical and Biophysical Research Communications. 311 (4): 979–986. doi:10.1016/j.bbrc.2003.10.094. ISSN 0006-291X. PMID 14623278.
- ^ Park, Enoch Y.; Ishikiriyama, Motoki; Nishina, Takuya; Kato, Tatsuya; Yagi, Hirokazu; Kato, Koichi; Ueda, Hiroshi (January 2009). "Human IgG1 expression in silkworm larval hemolymph using BmNPV bacmids and its N-linked glycan structure". Journal of Biotechnology. 139 (1): 108–114. doi:10.1016/j.jbiotec.2008.09.013. hdl:10297/5175. ISSN 0168-1656. PMID 18984019. S2CID 13070665.
- ^ Nomura, Tsuyoshi; Suganuma, Masatoshi; Higa, Yukiko; Kataoka, Yukiko; Funaguma, Shunsuke; Okazaki, Hironobu; Suzuki, Takeo; Kobayashi, Isao; Sezutsu, Hideki; Fujiyama, Kazuhito (February 2015). "Improvement of glycosylation structure by suppression of β-N-acetylglucosaminidases in silkworm". Journal of Bioscience and Bioengineering. 119 (2): 131–136. doi:10.1016/j.jbiosc.2014.07.012. ISSN 1389-1723. PMID 25193875.
- ^ Paschinger, Katharina; Yan, Shi; Wilson, Iain B. H. (2019-03-12). "N-glycomic Complexity in Anatomical Simplicity: Caenorhabditis elegans as a Non-model Nematode?". Frontiers in Molecular Biosciences. 6: 9. doi:10.3389/fmolb.2019.00009. ISSN 2296-889X. PMC 6422873. PMID 30915340.
- ^ Smith, Trevor S; Graham, Margaret; Munn, Edward A; Newton, Susan E; Knox, David P; Coadwell, W.John; McMichael-Phillips, Danielle; Smith, Howard; Smith, W.David; Oliver, Joanne J (April 1997). "Cloning and characterization of a microsomal aminopeptidase from the intestine of the nematode Haemonchus contortus". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1338 (2): 295–306. doi:10.1016/s0167-4838(96)00204-x. ISSN 0167-4838. PMID 9128148.
- ^ Pöltl, Gerald; Kerner, Denise; Paschinger, Katharina; Wilson, Iain B. H. (2006-12-20). "N-Glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues". FEBS Journal. 274 (3): 714–726. doi:10.1111/j.1742-4658.2006.05615.x. ISSN 1742-464X. PMC 2850173. PMID 17181538.
- ^ Hewitson, James P.; Filbey, Kara J.; Grainger, John R.; Dowle, Adam A.; Pearson, Mark; Murray, Janice; Harcus, Yvonne; Maizels, Rick M. (2011-09-30). "Heligmosomoides polygyrus Elicits a Dominant Nonprotective Antibody Response Directed against Restricted Glycan and Peptide Epitopes". The Journal of Immunology. 187 (9): 4764–4777. doi:10.4049/jimmunol.1004140. ISSN 0022-1767. PMC 4306209. PMID 21964031.
- ^ Wilson, Iain B. H.; Paschinger, Katharina (2015-12-09). "Sweet secrets of a therapeutic worm: mass-spectrometric N-glycomic analysis of Trichuris suis". Analytical and Bioanalytical Chemistry. 408 (2): 461–471. doi:10.1007/s00216-015-9154-8. ISSN 1618-2642. PMC 4712359. PMID 26650734.
- ^ Dam, Svend; Thaysen-Andersen, Morten; Stenkjær, Eva; Lorentzen, Andrea; Roepstorff, Peter; Packer, Nicolle H.; Stougaard, Jens (2013-06-25). "Combined N-Glycome and N-Glycoproteome Analysis of the Lotus japonicus Seed Globulin Fraction Shows Conservation of Protein Structure and Glycosylation in Legumes". Journal of Proteome Research. 12 (7): 3383–3392. doi:10.1021/pr400224s. ISSN 1535-3893. PMID 23799247.
- ^ Wang, Xianghong; Jiang, Daiming; Shi, Jingni; Yang, Daichang (January 2017). "Expression of α-1,6-fucosyltransferase (FUT8) in rice grain and immunogenicity evaluation of plant-specific glycans". Journal of Biotechnology. 242: 111–121. doi:10.1016/j.jbiotec.2016.12.017. ISSN 0168-1656. PMID 28013072.
- ^ Mathieu-Rivet, Elodie; Scholz, Martin; Arias, Carolina; Dardelle, Flavien; Schulze, Stefan; Le Mauff, François; Teo, Gavin; Hochmal, Ana Karina; Blanco-Rivero, Amaya; Loutelier-Bourhis, Corinne; Kiefer-Meyer, Marie-Christine (November 2013). "Exploring the N-glycosylation Pathway in Chlamydomonas reinhardtii Unravels Novel Complex Structures". Molecular & Cellular Proteomics. 12 (11): 3160–3183. doi:10.1074/mcp.m113.028191. ISSN 1535-9476. PMC 3820931. PMID 23912651.
- ^ HASE, Sumihiro; OKAWA, Kazunobu; IKENAKA, Tokuji (1982). "Identification of the Trimannosyl-Chitobiose Structure in Sugar Moieties of Japanese Quail Ovomucoid1". The Journal of Biochemistry. 91 (2): 735–737. doi:10.1093/oxfordjournals.jbchem.a133748. ISSN 1756-2651. PMID 7068587.
- ^ ALONSO, Josefa María; BOULENGUER, Patrick; WIERUSZESKI, Jean-Michel; LEROY, Yves; MONTREUIL, Jean; FOURNET, Bernard (2005-03-03). "Microheterogeneity and structures of neutral glycans present in quail ovomucoid". European Journal of Biochemistry. 177 (1): 187–197. doi:10.1111/j.1432-1033.1988.tb14361.x-i2. ISSN 0014-2956. PMID 3181154.
- ^ Faid, Valegh; Evjen, Gry; Tollersrud, Ole-Kristian; Michalski, Jean-Claude; Morelle, Willy (2006-01-31). "Site-specific glycosylation analysis of the bovine lysosomal α-mannosidase". Glycobiology. 16 (5): 440–461. doi:10.1093/glycob/cwj081. ISSN 1460-2423. PMID 16449350.
- ^ Howard, D R; Natowicz, M; Baenziger, J U (September 1982). "Structural studies of the endoglycosidase H-resistant oligosaccharides present on human beta-glucuronidase". Journal of Biological Chemistry. 257 (18): 10861–10868. doi:10.1016/s0021-9258(18)33904-8. ISSN 0021-9258. PMID 6809759. S2CID 24105430.
- ^ SUMIYOSHI, Wataru; NAKAKITA, Shin-ichi; HASEHIRA, Kayo; MIYANISHI, Nobumitsu; KUBO, Yuhki; KITA, Takayoshi; HIRABAYASHI, Jun (2010-03-23). "Comprehensive Analysis ofN-Linked Oligosaccharides from Eggs of the Family Phasianidae". Bioscience, Biotechnology, and Biochemistry. 74 (3): 606–613. doi:10.1271/bbb.90821. ISSN 0916-8451. PMID 20208342. S2CID 44957115.
- ^ Hanzawa, Ken; Suzuki, Noriko; Natsuka, Shunji (2016-12-08). "Structures and developmental alterations ofN-glycans of zebrafish embryos". Glycobiology. 27 (3): 228–245. doi:10.1093/glycob/cww124. ISSN 0959-6658. PMID 27932382.
- ^ Olczak, Mariusz; Wątorek, Wiesław (April 2002). "Structural analysis of N-glycans from human neutrophil azurocidin". Biochemical and Biophysical Research Communications. 293 (1): 213–219. doi:10.1016/s0006-291x(02)00201-2. ISSN 0006-291X. PMID 12054586.
- ^ Ravnsborg, Tina; Houen, Gunnar; Højrup, Peter (October 2010). "The glycosylation of myeloperoxidase". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1804 (10): 2046–2053. doi:10.1016/j.bbapap.2010.07.001. ISSN 1570-9639. PMID 20621206.
- ^ a b Tjondro, Harry C.; Ugonotti, Julian; Kawahara, Rebeca; Chatterjee, Sayantani; Loke, Ian; Chen, Siyun; Soltermann, Fabian; Hinneburg, Hannes; Parker, Benjamin L.; Venkatakrishnan, Vignesh; Dieckmann, Regis (January 2021). "Hyper-truncated Asn355- and Asn391-glycans modulate the activity of neutrophil granule myeloperoxidase". Journal of Biological Chemistry. 296: 100144. doi:10.1074/jbc.ra120.016342. ISSN 0021-9258. PMC 7857493. PMID 33273015.
- ^ a b Hinneburg, Hannes; Pedersen, Jessica L; Bokil, Nilesh J; Pralow, Alexander; Schirmeister, Falko; Kawahara, Rebeca; Rapp, Erdmann; Saunders, Bernadette M; Thaysen-Andersen, Morten (2020-03-09). "High-resolution longitudinal N- and O-glycoprofiling of human monocyte-to-macrophage transition". Glycobiology. 30 (9): 679–694. doi:10.1093/glycob/cwaa020. hdl:10453/146548. ISSN 1460-2423. PMID 32149347.
- ^ Parker, Robert; Partridge, Thomas; Wormald, Catherine; Kawahara, Rebeca; Stalls, Victoria; Aggelakopoulou, Maria; Parker, Jimmy; Powell Doherty, Rebecca; Ariosa Morejon, Yoanna; Lee, Esther; Saunders, Kevin; Haynes, Barton F.; Acharya, Priyamvada; Thaysen-Andersen, Morten; Borrow, Persephone; Ternette, Nicola (May 2021). "Mapping the SARS-CoV-2 spike glycoprotein-derived peptidome presented by HLA class II on dendritic cells". Cell Reports. 35 (8): 109179. bioRxiv 10.1101/2020.08.19.255901. doi:10.1016/j.celrep.2021.109179. PMC 8116342. PMID 34004174.
- ^ Yagi, H.; Nakagawa, M.; Takahashi, N.; Kondo, S.; Matsubara, M.; Kato, K. (2007-11-13). "Neural complex-specific expression of xylosyl N-glycan in Ciona intestinalis". Glycobiology. 18 (2): 145–151. doi:10.1093/glycob/cwm128. ISSN 0959-6658. PMID 18056652.
- ^ Ballou, Clinton E. (1990), "Isolation, characterization, and properties of Saccharomyces cerevisiae MNN mutants with nonconditional protein glycosylation defects", Gene Expression Technology, Methods in Enzymology, vol. 185, Elsevier, pp. 440–470, doi:10.1016/0076-6879(90)85038-p, ISBN 9780121820862, PMID 2199792
- ^ Masuoka, James (April 2004). "Surface Glycans Candida albicans and Other Pathogenic Fungi: Physiological Roles, Clinical Uses, and Experimental Challenges". Clinical Microbiology Reviews. 17 (2): 281–310. doi:10.1128/cmr.17.2.281-310.2004. ISSN 0893-8512. PMC 387410. PMID 15084502.
- ^ Altmann, Friedrich; Schwihla, Herwig; Staudacher, Erika; Glössl, Josef; März, Leopold (July 1995). "Insect Cells Contain an Unusual, Membrane-bound β-N-Acetylglucosaminidase Probably Involved in the Processing of Protein N-Glycans". Journal of Biological Chemistry. 270 (29): 17344–17349. doi:10.1074/jbc.270.29.17344. ISSN 0021-9258. PMID 7615537.
- ^ Rosenbaum, Erica E.; Vasiljevic, Eva; Brehm, Kimberley S.; Colley, Nansi Jo (2014-05-01). "Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in Drosophila melanogaster". PLOS Genetics. 10 (5): e1004349. doi:10.1371/journal.pgen.1004349. ISSN 1553-7404. PMC 4006722. PMID 24785692. S2CID 5699432.
- ^ Cattaneo, F.; Pasini, M. E.; Intra, J.; Matsumoto, M.; Briani, F.; Hoshi, M.; Perotti, M. E. (2006-05-29). "Identification and expression analysis of Drosophilamelanogaster genes encoding β-hexosaminidases of the sperm plasma membrane". Glycobiology. 16 (9): 786–800. doi:10.1093/glycob/cwl007. ISSN 1460-2423. PMID 16733265.
- ^ Cattaneo, F.; Ogiso, M.; Hoshi, M.; Perotti, M.-E.; Pasini, M.E. (August 2002). "Purification and characterization of the plasma membrane glycosidases of Drosophila melanogaster spermatozoa". Insect Biochemistry and Molecular Biology. 32 (8): 929–941. Bibcode:2002IBMB...32..929C. doi:10.1016/s0965-1748(02)00031-0. ISSN 0965-1748. PMID 12110300.
- ^ Gutternigg, Martin; Kretschmer-Lubich, Dorothea; Paschinger, Katharina; Rendić, Dubravko; Hader, Josef; Geier, Petra; Ranftl, Ramona; Jantsch, Verena; Lochnit, Günter; Wilson, Iain B.H. (September 2007). "Biosynthesis of Truncated N-Linked Oligosaccharides Results from Non-orthologous Hexosaminidase-mediated Mechanisms in Nematodes, Plants, and Insects". Journal of Biological Chemistry. 282 (38): 27825–27840. doi:10.1074/jbc.m704235200. ISSN 0021-9258. PMC 2850174. PMID 17636254.
- ^ Paschinger, Katharina; Gutternigg, Martin; Rendić, Dubravko; Wilson, Iain B.H. (August 2008). "The N-glycosylation pattern of Caenorhabditis elegans". Carbohydrate Research. 343 (12): 2041–2049. doi:10.1016/j.carres.2007.12.018. ISSN 0008-6215. PMID 18226806.
- ^ Igarashi, Kiyohiko; Wada, Masahisa; Samejima, Masahiro (2009). "Kinetic Analysis of Cellobiohydrolase: Quantification of Enzymatic Reaction at a Solid/Liquid Interface Applying the Concept of Surface Density". Trends in Glycoscience and Glycotechnology. 21 (117): 13–22. doi:10.4052/tigg.21.13. ISSN 0915-7352.
- ^ Yan, Shi; Wang, Huijie; Schachter, Harry; Jin, Chunsheng; Wilson, Iain B.H.; Paschinger, Katharina (October 2018). "Ablation of N-acetylglucosaminyltransferases in Caenorhabditis induces expression of unusual intersected and bisected N-glycans". Biochimica et Biophysica Acta (BBA) - General Subjects. 1862 (10): 2191–2203. doi:10.1016/j.bbagen.2018.07.002. ISSN 0304-4165. PMC 6173287. PMID 29981898.
- ^ Shin, Yun-Ji; Castilho, Alexandra; Dicker, Martina; Sádio, Flavio; Vavra, Ulrike; Grünwald-Gruber, Clemens; Kwon, Tae-Ho; Altmann, Friedrich; Steinkellner, Herta; Strasser, Richard (2016-08-11). "Reduced paucimannosidicN-glycan formation by suppression of a specific β-hexosaminidase fromNicotiana benthamiana". Plant Biotechnology Journal. 15 (2): 197–206. doi:10.1111/pbi.12602. ISSN 1467-7644. PMC 5259580. PMID 27421111.
- ^ Strasser, Richard; Bondili, Jayakumar Singh; Schoberer, Jennifer; Svoboda, Barbara; Liebminger, Eva; Glössl, Josef; Altmann, Friedrich; Steinkellner, Herta; Mach, Lukas (2007-07-20). "Enzymatic Properties and Subcellular Localization of Arabidopsis β-N-Acetylhexosaminidases". Plant Physiology. 145 (1): 5–16. doi:10.1104/pp.107.101162. ISSN 1532-2548. PMC 1976588. PMID 17644627. S2CID 13573193.
- ^ a b Pedersen, Carina T.; Loke, Ian; Lorentzen, Andrea; Wolf, Sara; Kamble, Manoj; Kristensen, Sebastian K.; Munch, David; Radutoiu, Simona; Spillner, Edzard; Roepstorff, Peter; Thaysen-Andersen, Morten (2017-05-22). "N-glycan maturation mutants in Lotus japonicus for basic and applied glycoprotein research". The Plant Journal. 91 (3): 394–407. doi:10.1111/tpj.13570. ISSN 0960-7412. PMID 28407380. S2CID 6370095.
- ^ Strasser, Richard; Schoberer, Jennifer; Jin, Chunsheng; Glössl, Josef; Mach, Lukas; Steinkellner, Herta (March 2006). "Molecular cloning and characterization ofArabidopsis thalianaGolgi α-mannosidase II, a key enzyme in the formation of complex N-glycans in plants". The Plant Journal. 45 (5): 789–803. doi:10.1111/j.1365-313x.2005.02648.x. ISSN 0960-7412. PMID 16460512.
- ^ Strasser, R.; Altmann, F.; Mach, L.; Glössl, J.; Steinkellner, H. (2004-02-20). "Generation of Arabidopsis thaliana plants with complex N-glycans lacking β1,2-linked xylose and core α1,3-linked fucose". FEBS Letters. 561 (1–3): 132–136. Bibcode:2004FEBSL.561..132S. doi:10.1016/s0014-5793(04)00150-4. ISSN 0014-5793. PMID 15013764. S2CID 83647511.
- ^ Ghosh, Sumit; Meli, Vijaykumar S.; Kumar, Anil; Thakur, Archana; Chakraborty, Niranjan; Chakraborty, Subhra; Datta, Asis (2010-10-28). "The N-glycan processing enzymes α-mannosidase and β-D-N-acetylhexosaminidase are involved in ripening-associated softening in the non-climacteric fruits of capsicum". Journal of Experimental Botany. 62 (2): 571–582. doi:10.1093/jxb/erq289. ISSN 1460-2431. PMC 3003805. PMID 21030387.
- ^ a b Hepbildikler, Stefan T.; Sandhoff, Roger; Kölzer, Melanie; Proia, Richard L.; Sandhoff, Konrad (January 2002). "Physiological Substrates for Human Lysosomal β-Hexosaminidase S". Journal of Biological Chemistry. 277 (4): 2562–2572. doi:10.1074/jbc.m105457200. ISSN 0021-9258. PMID 11707436.
- ^ Cowland, Jack B.; Borregaard, Niels (2016-08-25). "Granulopoiesis and granules of human neutrophils". Immunological Reviews. 273 (1): 11–28. doi:10.1111/imr.12440. ISSN 0105-2896. PMID 27558325. S2CID 28294497.
- ^ Venkatakrishnan, Vignesh; Dieckmann, Regis; Loke, Ian; Tjondro, Harry; Chatterjee, Sayantani; Bylund, Johan; Thaysen-Andersen, Morten; Karlsson, Niclas G.; Karlsson-Bengtsson, Anna (2020-04-02). "Glycan analysis of human neutrophil granules implicates a maturation-dependent glycosylation machinery". The Journal of Biological Chemistry. 295 (36): 12648–12660. bioRxiv 10.1101/2020.04.02.021394. doi:10.1074/jbc.RA120.014011. PMC 7476722. PMID 32665399. S2CID 215403886.
- ^ a b c M, Hare, NJ Lee, LY Loke, I Britton, WJ Saunders, BM Thaysen-Andersen (2017-01-06). Mycobacterium tuberculosis Infection Manipulates the Glycosylation Machinery and the N-Glycoproteome of Human Macrophages and Their Microparticles. OCLC 1105695513.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Robajac, Dragana; Vanhooren, Valerie; Masnikosa, Romana; Miković, Željko; Mandić, Vesna; Libert, Claude; Nedić, Olgica (February 2016). "Preeclampsia transforms membrane N-glycome in human placenta". Experimental and Molecular Pathology. 100 (1): 26–30. doi:10.1016/j.yexmp.2015.11.029. ISSN 0014-4800. PMID 26655437.
- ^ Pak, Joanne; Pu, Yongbing; Zhang, Zhong-Ting; Hasty, David L.; Wu, Xue-Ru (March 2001). "Tamm-Horsfall Protein Binds to Type 1 Fimbriated Escherichia coli and Prevents E. coli from Binding to Uroplakin Ia and Ib Receptors". Journal of Biological Chemistry. 276 (13): 9924–9930. doi:10.1074/jbc.m008610200. ISSN 0021-9258. PMID 11134021.
- ^ Venkatakrishnan, Vignesh; Thaysen-Andersen, Morten; Chen, Sharon C A; Nevalainen, Helena; Packer, Nicolle H (2014-09-04). "Cystic fibrosis and bacterial colonization define the sputum N-glycosylation phenotype". Glycobiology. 25 (1): 88–100. doi:10.1093/glycob/cwu092. ISSN 0959-6658. PMID 25190359.
- ^ Everest-Dass, Arun V; Jin, Dayong; Thaysen-Andersen, Morten; Nevalainen, Helena; Kolarich, Daniel; Packer, Nicolle H (2012-07-24). "Comparative structural analysis of the glycosylation of salivary and buccal cell proteins: innate protection against infection by Candida albicans". Glycobiology. 22 (11): 1465–1479. doi:10.1093/glycob/cws112. ISSN 1460-2423. PMID 22833316.
- ^ Loke, Ian; Packer, Nicolle; Thaysen-Andersen, Morten (2015-08-12). "Complementary LC-MS/MS-Based N-Glycan, N-Glycopeptide, and Intact N-Glycoprotein Profiling Reveals Unconventional Asn71-Glycosylation of Human Neutrophil Cathepsin G". Biomolecules. 5 (3): 1832–1854. doi:10.3390/biom5031832. ISSN 2218-273X. PMC 4598777. PMID 26274980.
- ^ Becker, Yvonne; Förster, Sarah; Gielen, Gerrit H.; Loke, Ian; Thaysen-Andersen, Morten; Laurini, Christine; Wehrand, Kristin; Pietsch, Torsten; Diestel, Simone (2019-07-08). "Paucimannosidic glycoepitopes inhibit tumorigenic processes in glioblastoma multiforme". Oncotarget. 10 (43): 4449–4465. doi:10.18632/oncotarget.27056. ISSN 1949-2553. PMC 6633888. PMID 31320997.
- ^ Lee, Ling Y.; Thaysen-Andersen, Morten; Baker, Mark S.; Packer, Nicolle H.; Hancock, William S.; Fanayan, Susan (2014-09-11). "Comprehensive N-Glycome Profiling of Cultured Human Epithelial Breast Cells Identifies Unique Secretome N-Glycosylation Signatures Enabling Tumorigenic Subtype Classification". Journal of Proteome Research. 13 (11): 4783–4795. doi:10.1021/pr500331m. ISSN 1535-3893. PMID 25210975.
- ^ Abrahams, Jodie L.; Campbell, Matthew P.; Packer, Nicolle H. (2017-09-13). "Building a PGC-LC-MS N-glycan retention library and elution mapping resource". Glycoconjugate Journal. 35 (1): 15–29. doi:10.1007/s10719-017-9793-4. ISSN 0282-0080. PMID 28905148. S2CID 3885305.
- ^ "Squamous cell carcinoma and basal cell carcinoma of the skin", Textbook of Surgical Oncology, CRC Press, pp. 327–334, 2007-11-15, doi:10.3109/9780203003220-30, ISBN 978-0-429-21406-6
- ^ Hinneburg, Hannes; Korać, Petra; Schirmeister, Falko; Gasparov, Slavko; Seeberger, Peter H.; Zoldoš, Vlatka; Kolarich, Daniel (April 2017). "Unlocking Cancer Glycomes from Histopathological Formalin-fixed and Paraffin-embedded (FFPE) Tissue Microdissections". Molecular & Cellular Proteomics. 16 (4): 524–536. doi:10.1074/mcp.m116.062414. ISSN 1535-9476. PMC 5383776. PMID 28122943.
- ^ Everest-Dass, Arun V.; Briggs, Matthew T.; Kaur, Gurjeet; Oehler, Martin K.; Hoffmann, Peter; Packer, Nicolle H. (September 2016). "N-glycan MALDI Imaging Mass Spectrometry on Formalin-Fixed Paraffin-Embedded Tissue Enables the Delineation of Ovarian Cancer Tissues". Molecular & Cellular Proteomics. 15 (9): 3003–3016. doi:10.1074/mcp.m116.059816. ISSN 1535-9476. PMC 5013313. PMID 27412689.
- ^ Kawahara, Rebeca; Ortega, Fabio; Rosa-Fernandes, Livia; Guimarães, Vanessa; Quina, Daniel; Nahas, Willian; Schwämmle, Veit; Srougi, Miguel; Leite, Katia R.M.; Thaysen-Andersen, Morten; Larsen, Martin R. (2018-09-04). "Distinct urinary glycoprotein signatures in prostate cancer patients". Oncotarget. 9 (69): 33077–33097. doi:10.18632/oncotarget.26005. ISSN 1949-2553. PMC 6145689. PMID 30237853.
- ^ Jarmo, Satomaa, Tero Heiskanen, Annamari Mikkola, Milla Olsson, Cia Blomqvist, Maria Tiittanen, Minna Jaatinen, Taina Aitio, Olli Olonen, Anne Helin, Jari Hiltunen, Jukka Natunen, Jari Tuuri, Timo Otonkoski, Timo Saarinen, Juhani Laine (2009-06-02). The N-glycome of human embryonic stem cells. BioMed Central Ltd. OCLC 808635211.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Dahmen, Ann-Christine; Fergen, Marie-Therese; Laurini, Christine; Schmitz, Brigitte; Loke, Ian; Thaysen-Andersen, Morten; Diestel, Simone (2015-04-28). "Paucimannosidic glycoepitopes are functionally involved in proliferation of neural progenitor cells in the subventricular zone". Glycobiology. 25 (8): 869–880. doi:10.1093/glycob/cwv027. ISSN 0959-6658. PMID 25922361.
- ^ Taganna, Joemar; de Boer, Arjen R.; Wuhrer, Manfred; Bouckaert, Julie (2011-01-19). "Glycosylation changes as important factors for the susceptibility to urinary tract infection". Biochemical Society Transactions. 39 (1): 349–354. doi:10.1042/bst0390349. ISSN 0300-5127. PMID 21265802.
- ^ Marotte, Karine; Sabin, Charles; Préville, Cathy; Moumé-Pymbock, Myriam; Wimmerová, Michaela; Mitchell, Edward P.; Imberty, Anne; Roy, René (2007-09-10). "X-ray Structures and Thermodynamics of the Interaction of PA-IIL fromPseudomonas aeruginosa with Disaccharide Derivatives". ChemMedChem. 2 (9): 1328–1338. doi:10.1002/cmdc.200700100. ISSN 1860-7179. PMID 17623286. S2CID 9113181.
- ^ Léonard, Renaud; Rendić, Dubravko; Rabouille, Catherine; Wilson, Iain B.H.; Préat, Thomas; Altmann, Friedrich (February 2006). "The Drosophila fused lobes Gene Encodes an N-Acetylglucosaminidase Involved in N-Glycan Processing". Journal of Biological Chemistry. 281 (8): 4867–4875. doi:10.1074/jbc.m511023200. ISSN 0021-9258. PMID 16339150.
- ^ Mortimer, Nathan T.; Kacsoh, Balint Z.; Keebaugh, Erin S.; Schlenke, Todd A. (2012-07-19). "Mgat1-dependent N-glycosylation of Membrane Components Primes Drosophila melanogaster Blood Cells for the Cellular Encapsulation Response". PLOS Pathogens. 8 (7): e1002819. doi:10.1371/journal.ppat.1002819. ISSN 1553-7374. PMC 3400557. PMID 22829770.
- ^ Walski, Tomasz; Van Damme, Els J. M.; Smargiasso, Nicolas; Christiaens, Olivier; De Pauw, Edwin; Smagghe, Guy (2016-10-12). "Protein N-glycosylation and N-glycan trimming are required for postembryonic development of the pest beetle Tribolium castaneum". Scientific Reports. 6 (1): 35151. Bibcode:2016NatSR...635151W. doi:10.1038/srep35151. ISSN 2045-2322. PMC 5059678. PMID 27731363.
- ^ Cipollo, John F.; Awad, Antoine M.; Costello, Catherine E.; Hirschberg, Carlos B. (July 2005). "N-Glycans of Caenorhabditis elegans Are Specific to Developmental Stages". Journal of Biological Chemistry. 280 (28): 26063–26072. doi:10.1074/jbc.m503828200. ISSN 0021-9258. PMID 15899899.
- ^ Yan, Shi; Bleuler-Martinez, Silvia; Plaza, David Fernando; Künzler, Markus; Aebi, Markus; Joachim, Anja; Razzazi-Fazeli, Ebrahim; Jantsch, Verena; Geyer, Rudolf; Wilson, Iain B.H.; Paschinger, Katharina (August 2012). "Galactosylated Fucose Epitopes in Nematodes". Journal of Biological Chemistry. 287 (34): 28276–28290. doi:10.1074/jbc.m112.353128. ISSN 0021-9258. PMC 3436517. PMID 22733825.
- ^ Hewitson, James P.; Harcus, Yvonne M.; Curwen, Rachel S.; Dowle, Adam A.; Atmadja, Agnes K.; Ashton, Peter D.; Wilson, Alan; Maizels, Rick M. (July 2008). "The secretome of the filarial parasite, Brugia malayi: Proteomic profile of adult excretory–secretory products". Molecular and Biochemical Parasitology. 160 (1): 8–21. doi:10.1016/j.molbiopara.2008.02.007. ISSN 0166-6851. PMID 18439691.
- ^ Shi, Hui; Tan, Jenny; Schachter, Harry (2006), "N-Glycans Are Involved in the Response of Caenorhabditis elegans to Bacterial Pathogens", Functional Glycomics, Methods in Enzymology, vol. 417, Elsevier, pp. 359–389, doi:10.1016/s0076-6879(06)17022-6, ISBN 9780121828226, PMID 17132514
This article needs additional or more specific categories. (August 2022) |
