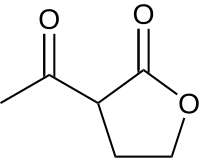
2-Acetylbutyrolactone
| |
| Names | |
|---|---|
| IUPAC name
3-acetyloxolan-2-one
| |
| Other names
3-acetyldihydrofuran-2(3H)-one
2-acetyl-γ-butyrolactone | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | ABL |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.488 |
| EC Number |
|
| MeSH | 2-acetylbutyrolactone |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8O3 | |
| Molar mass | 128.127 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 1.19 g/cm3 [1] |
| Boiling point | 107–108 °C (225–226 °F; 380–381 K) (at 7 hPa)[1] |
| Solubility | Soluble in DMF,[2] methanol[3] |
| Hazards | |
| GHS labelling:[4] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P271, P280, P302+P352, P304+P340, P305+P351+P338, P321, P362+P364, P403+P233, P405, P501 | |
| Flash point | 113 °C (235 °F; 386 K)[1] |
| Safety data sheet (SDS) | Sigma-Aldrich SDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Acetylbutyrolactone (ABL) is a derivative of γ-butyrolactone that is used as a precursor in organic synthesis, and it is used to identify primary amines through chemical fluorescence.[2]
Preparation
[edit]2-Acetylbutyrolactone can be prepared by a condensation reaction between an ester of acetic acid (such as ethyl acetate) with butyrolactone in an alkaline solution.[5]
2-Acetylbutyrolactone can also be prepared by reacting ethylene oxide with ethyl acetoacetate in alkaline conditions.[citation needed]
Uses
[edit]Spectrofluorimetry
[edit]2-Acetylbutyrolactone itself is only slightly fluorescent, but its derivatives show high UV fluorescence.[3][6] The carbonyl group readily reacts with amines to form Schiff bases. It is for this reason that 2-acetylbutyrolactone is frequently used to confirm the creation of amines during organic synthesis.[3] 2-Acetylbutyrolactone can also undergo a Japp–Klingemann reaction to form fluorescent molecules with arylamines.[6]
Drug precursor
[edit]Uses of 2-Acetylbutyrolactone also includes synthesis of:
- Risperidone
- Ritanserin
- Paliperidone
- Ocaperidone
- Seganserin
- Setoperone
- Metrenperone
- Pirenperone
- Novoldiamine (1-Diethylamino-4-aminopentane) [140-80-7]
- Clomethiazole
- Barmastine
- R 59-022 [93076-89-2]
- ID-4708 [42048-72-6].
- Santalene[7]
- α-methylene-γ-butyrolactones.[8]
References
[edit]- ^ a b c "α-Acetylbutyrolactone Safety Data Sheet". SigmaAldrich. January 20, 2020. Archived from the original on 2022-03-31. Retrieved March 30, 2022.
- ^ a b Sabry, Suzy M. (2006). "Application of 2-acetylbutyrolactone to spectrofluorimetry: Fluorescence properties of Schiff bases derived from 2-acetylbutyrolactone and spectrofluorimetric determination of primary amine-containing compounds". Journal of Pharmaceutical and Biomedical Analysis. 40 (5): 1057–1067. doi:10.1016/j.jpba.2005.08.036. PMID 16256289.
- ^ a b c Sabry, S M (2006). "Application of 2-acetylbutyrolactone to spectrofluorimetry: Fluorescence properties of Schiff bases derived from 2-acetylbutyrolactone and spectrofluorimetric determination of primary amine-containing compounds". Journal of Pharmaceutical and Biomedical Analysis. 40 (5): 1057–1067. doi:10.1016/j.jpba.2005.08.036. PMID 16256289.
- ^ "2-Acetylbutyrolactone". pubchem.ncbi.nlm.nih.gov. Retrieved 31 March 2022.
- ^ Koehler, Guenther (August 4, 1998). "Method for Preparing 2-Acetyl-y-Butyrolactone". United States Patent. Archived from the original on 2022-03-31. Retrieved March 30, 2022.
- ^ a b Sabry, S M (2006). "Enhanced Spectrophotometry of Sulfonamides with Novel 2-Acetylbutyrolactone Derivatives". Analytical Letters. 39 (13): 2591–2615. doi:10.1080/00032710600824748. S2CID 93950011.
- ^ Unnikrishnan, P. A.; Vatakencherry, P. A. (1992). "Syntheses of epi-β-Santalene, β-Santalene and an Isomer of β-Santalene with 4-Methyl-4-pentenyl Side Chain". Synthetic Communications. 22 (22): 3159–3168. doi:10.1080/00397919208021129.
- ^ Aghari, S (2006). "Reaction of tert-butyl isocyanide and dialkyl acetylenedicarboxylates in the presence of 2-acetylbutyrolactone. Synthesis of functionalized α-methylene-γ-butyrolactones". Tetrahedron. 47 (25): 4297–4299. doi:10.1016/j.tetlet.2006.03.109.
