Jelly roll fold

The jelly roll or Swiss roll fold is a protein fold or supersecondary structure composed of eight beta strands arranged in two four-stranded sheets. The name of the structure was introduced by Jane S. Richardson in 1981, reflecting its resemblance to the jelly or Swiss roll cake.[2] The fold is an elaboration on the Greek key motif and is sometimes considered a form of beta barrel. It is very common in viral proteins, particularly viral capsid proteins.[3][4] Taken together, the jelly roll and Greek key structures comprise around 30% of the all-beta proteins annotated in the Structural Classification of Proteins (SCOP) database.[5]
Structure
[edit]The basic jelly roll structure consists of eight beta strands arranged in two four-stranded antiparallel beta sheets which pack together across a hydrophobic interface [Where citation... uniprot]. The strands are traditionally labeled B through I for the historical reason that the first solved structure, of a jelly roll capsid protein from the tomato bushy stunt virus, had an additional strand A outside the fold's common core.[6][7] The sheets are composed of strands BIDG and CHEF, folded such that strand B packs opposite strand C, I opposite H, etc.[4][8]
Viral proteins
[edit]
A large number of viruses build their exterior capsids from proteins containing either a single or a double jelly roll fold. This shared capsid architecture is thought to reflect ancient evolutionary relationships, possibly dating to before the last universal common ancestor (LUCA) of cellular life.[8][9][10] Other viral lineages use evolutionarily unrelated proteins to build their enclosed capsids, which likely evolved independently at least twice[9][11] and possibly many times, with links to proteins of cellular origin.[12]
Single jelly roll capsid proteins
[edit]Single jelly roll capsid (JRC) proteins are found in at least sixteen distinct viral families, mostly with icosahedral capsid structures and including both RNA viruses and DNA viruses.[13] Many viruses with single jelly roll capsids are positive-sense single-stranded RNA viruses. Two groups of double-stranded DNA viruses with single-JRC capsids are the Papillomaviridae and Polyomaviridae, both of which have fairly small capsids; in these viruses, the architecture of the assembled capsid orients the axis of the jelly roll parallel or "horizontally" relative to the capsid surface.[11] A large-scale analysis of viral capsid components suggested that the single horizontal jelly roll is the most common fold among capsid proteins, accounting for about 28% of known examples.[12]
Another group of viruses uses single jelly roll proteins in their capsids, but in the vertical rather than horizontal orientation. These viruses are evolutionarily related to the large group of double jelly-roll viruses known as the PRD1-adenovirus viral lineage, with similar capsid architecture realized through assembly of two distinct single jelly-roll major capsid proteins expressed from distinct genes.[14][15] These single vertical jelly-roll viruses comprise the taxon Helvetiavirae.[16] Known viruses with vertical single jelly roll capsids infect extremophilic prokaryotes.[14][12]
Double jelly roll proteins
[edit]
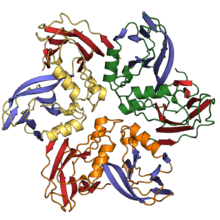
Double jelly roll capsid proteins consist of two single jelly roll folds connected by a short linker region. They are found in both double-stranded DNA viruses and single-stranded DNA viruses of at least ten different viral families, including viruses that infect all domains of life, and spanning a large capsid size range.[4][11][18] In the double jelly roll capsid architecture, the jelly roll axis is oriented perpendicular or "vertically" relative to the capsid surface.[19]
Double jelly roll proteins are believed to have evolved from single jelly roll proteins by gene duplication.[11][19] It is likely that vertical single jelly roll viruses represent a transitional form, and that the vertical and horizontal jelly roll capsid proteins have independent evolutionary origins from ancestral cellular proteins.[12] The degree of structural similarity among double-jelly-roll virus capsids has led to the conclusion that these viruses likely have a common evolutionary origin despite their diversity in size and in host range; this has become known as the PRD1-adenovirus lineage (Bamfordvirae).[19][16][20][21] Many members of this group have been identified through metagenomics and in some cases have few to no other viral genes in common.[12][22] Although most members of this group have icosahedral capsid geometry, a few families such as the Poxviridae and Ascoviridae have oval or brick-shaped mature virions; poxviruses such as Vaccinia undergo dramatic conformational changes mediated by highly derived double jelly roll proteins during maturation and likely derive from an icosahedral ancestor.[11][23] Shared double-jelly-roll capsid proteins, along with other homologous proteins, have also been cited in support of the proposed order Megavirales containing the nucleocytoplasmic large DNA viruses (NCLDV).[24]
Initially, it was believed that double jelly roll proteins are unique to viruses, because they were not observed in cellular proteins.[11] However, in 2022, comparison of protein structures revealed several families of bona fide cellular proteins with the double jelly roll fold [25]
Non-capsid proteins
[edit]Single jelly rolls also occur in non-capsid viral proteins, including minor components of the assembled virion as well as non-virion proteins such as polyhedrin.[11] In plant viruses, the 30K superfamily movement proteins responsible for intercellular transport of viral genomes or entire capsids through plasmodesmata channels have the single jelly-roll fold and have evolved from the capsid proteins of small icosahedral viruses.[26]
Cellular proteins
[edit]Both single and double jelly roll folds are found in proteins of cellular origin.[11][12][25] One class of cellular proteins with single jelly roll fold is the nucleoplasmins, which serve as molecular chaperone proteins for histone assembly into nucleosomes. The N-terminal domain of nucleoplasmins possesses a single jelly roll fold and assembled into a pentamer.[27] Similar structures have since been reported in additional groups of chromatin remodeling proteins.[28] Jelly roll motifs with identical beta-sheet connectivity are also found in tumor necrosis factor ligands[29] and proteins from the bacterium Yersinia pseudotuberculosis that belong to a class of viral and bacterial proteins known as superantigens.[30][31]
More broadly, the members of the extremely diverse cupin superfamily are also often described as jelly rolls; though the common core of the cupin domain structure contains only six beta strands, many cupins have eight.[32] Examples include the non-heme dioxygenase enzymes[33][34] (including alpha-ketoglutarate-dependent hydroxylases) and JmjC-family histone demethylases.[35][36]
Cellular proteins with the double jelly roll fold include glycoside hydrolases of the DUF2961 family, peptide:N-glycosidase F (PNGases F) and peptidylglycine alpha-amidating monooxygenase.[25]

A notable difference between PNGases F and the other double jelly roll proteins is the absence of the α-helices, which follow the F and F' strands in capsid proteins and DUF2961. The equivalent regions are variable in the PNGases F and contain either long loops or insertions. By contrast, jelly-roll domains of DUF2961 proteins contain an insertion of short β-hairpins upstream of the G and G' strands of the double jelly roll fold. Importantly, DUF2961 family proteins form trimers resembling viral capsomers.[25]
Evolution
[edit]Comparative studies of proteins classified as jelly roll and Greek key structures suggest that the Greek key proteins evolved significantly earlier than their more topologically complex jelly roll counterparts.[5] Structural bioinformatics studies comparing virus capsid jelly-roll proteins to other proteins of known structure indicates that the capsid proteins form a well-separated cluster, suggesting that they are subject to a distinctive set of evolutionary constraints.[4] One of the most notable features of viral capsid jelly roll proteins is their ability to form oligomers in a repeated tiling pattern to produce a closed protein shell; the cellular proteins that are most similar in fold and topology are mostly also oligomers.[4] It has been proposed that viral jelly-roll capsid proteins have evolved from cellular jelly-roll proteins, potentially on several independent occasions, at the earliest stages of cellular evolution.[12]
History and nomenclature
[edit]The name "jelly roll" was first used for the structure composed of an elaboration on the Greek key motif by Jane S. Richardson in 1981 and was intended to reflect the structure's resemblance to a jelly or Swiss roll cake.[2] The structure has been given a variety of descriptive names, including a wedge, beta barrel, and beta roll. The edges of the two sheets do not meet to form regular hydrogen bonding patterns, and so it is often not considered to be a true beta barrel,[3] though the term is in common use in describing viral capsid architecture.[14][15] Cellular proteins containing jelly roll-like structures may be described as a cupin fold, a JmjC fold, or a double-stranded beta helix.[34]
References
[edit]- ^ a b Larson SB, Day JS, McPherson A (September 2014). "Satellite tobacco mosaic virus refined to 1.4 Å resolution". Acta Crystallographica. Section D, Biological Crystallography. 70 (Pt 9): 2316–30. doi:10.1107/S1399004714013789. PMC 4157444. PMID 25195746.
- ^ a b Richardson JS (1981). "The anatomy and taxonomy of protein structure". Advances in Protein Chemistry Volume 34. Vol. 34. pp. 167–339. doi:10.1016/S0065-3233(08)60520-3. ISBN 9780120342341. PMID 7020376.
- ^ a b Chelvanayagam G, Heringa J, Argos P (November 1992). "Anatomy and evolution of proteins displaying the viral capsid jellyroll topology". Journal of Molecular Biology. 228 (1): 220–42. doi:10.1016/0022-2836(92)90502-B. PMID 1447783.
- ^ a b c d e Cheng S, Brooks CL (7 February 2013). "Viral capsid proteins are segregated in structural fold space". PLOS Computational Biology. 9 (2): e1002905. Bibcode:2013PLSCB...9E2905C. doi:10.1371/journal.pcbi.1002905. PMC 3567143. PMID 23408879.
- ^ a b Edwards H, Abeln S, Deane CM (14 November 2013). "Exploring fold space preferences of new-born and ancient protein superfamilies". PLOS Computational Biology. 9 (11): e1003325. Bibcode:2013PLSCB...9E3325E. doi:10.1371/journal.pcbi.1003325. PMC 3828129. PMID 24244135.
- ^ Harrison SC, Olson AJ, Schutt CE, Winkler FK, Bricogne G (November 1978). "Tomato bushy stunt virus at 2.9 A resolution". Nature. 276 (5686): 368–73. Bibcode:1978Natur.276..368H. doi:10.1038/276368a0. PMID 19711552. S2CID 4341051.
- ^ Rossmann MG, Abad-Zapatero C, Murthy MR, Liljas L, Jones TA, Strandberg B (April 1983). "Structural comparisons of some small spherical plant viruses". Journal of Molecular Biology. 165 (4): 711–36. doi:10.1016/S0022-2836(83)80276-9. PMID 6854630.
- ^ a b Benson SD, Bamford JK, Bamford DH, Burnett RM (December 2004). "Does common architecture reveal a viral lineage spanning all three domains of life?". Molecular Cell. 16 (5): 673–85. doi:10.1016/j.molcel.2004.11.016. PMID 15574324.
- ^ a b Forterre P, Prangishvili D (September 2009). "The origin of viruses". Research in Microbiology. 160 (7): 466–72. doi:10.1016/j.resmic.2009.07.008. PMID 19647075. S2CID 2767388.
- ^ Holmes EC (June 2011). "What does virus evolution tell us about virus origins?". Journal of Virology. 85 (11): 5247–51. doi:10.1128/JVI.02203-10. PMC 3094976. PMID 21450811.
- ^ a b c d e f g h Krupovic M, Bamford DH (August 2011). "Double-stranded DNA viruses: 20 families and only five different architectural principles for virion assembly". Current Opinion in Virology. 1 (2): 118–24. doi:10.1016/j.coviro.2011.06.001. PMID 22440622.
- ^ a b c d e f g Krupovic M, Koonin EV (March 2017). "Multiple origins of viral capsid proteins from cellular ancestors". Proceedings of the National Academy of Sciences of the United States of America. 114 (12): E2401–E2410. Bibcode:2017PNAS..114E2401K. doi:10.1073/pnas.1621061114. PMC 5373398. PMID 28265094.
- ^ Krupovic M (October 2013). "Networks of evolutionary interactions underlying the polyphyletic origin of ssDNA viruses". Current Opinion in Virology. 3 (5): 578–86. doi:10.1016/j.coviro.2013.06.010. PMID 23850154.
- ^ a b c Gil-Carton D, Jaakkola ST, Charro D, Peralta B, Castaño-Díez D, Oksanen HM, et al. (October 2015). "Insight into the Assembly of Viruses with Vertical Single β-barrel Major Capsid Proteins". Structure. 23 (10): 1866–1877. doi:10.1016/j.str.2015.07.015. PMID 26320579.
- ^ a b Santos-Pérez I, Charro D, Gil-Carton D, Azkargorta M, Elortza F, Bamford DH, et al. (March 2019). "Structural basis for assembly of vertical single β-barrel viruses". Nature Communications. 10 (1): 1184. Bibcode:2019NatCo..10.1184S. doi:10.1038/s41467-019-08927-2. PMC 6414509. PMID 30862777.
- ^ a b Koonin EV, Dolja VV, Krupovic M, Varsani A, Wolf YI, Yutin N, Zerbini M, Kuhn JH (October 2019). "Create a megataxonomic framework, filling all principal taxonomic ranks, for DNA viruses encoding vertical jelly roll-type major capsid proteins". ICTV Proposal (Taxoprop): 2019.003G. doi:10.13140/RG.2.2.14886.47684.
- ^ a b Abrescia NG, Grimes JM, Kivelä HM, Assenberg R, Sutton GC, Butcher SJ, et al. (September 2008). "Insights into virus evolution and membrane biogenesis from the structure of the marine lipid-containing bacteriophage PM2". Molecular Cell. 31 (5): 749–61. doi:10.1016/j.molcel.2008.06.026. PMID 18775333.
- ^ Laanto E, Mäntynen S, De Colibus L, Marjakangas J, Gillum A, Stuart DI, et al. (August 2017). "Virus found in a boreal lake links ssDNA and dsDNA viruses". Proceedings of the National Academy of Sciences of the United States of America. 114 (31): 8378–8383. Bibcode:2017PNAS..114.8378L. doi:10.1073/pnas.1703834114. PMC 5547622. PMID 28716906.
- ^ a b c Krupovic M, Bamford DH (December 2008). "Virus evolution: how far does the double beta-barrel viral lineage extend?". Nature Reviews. Microbiology. 6 (12): 941–8. doi:10.1038/nrmicro2033. PMID 19008892. S2CID 31542714.
- ^ Koonin EV, Dolja VV, Krupovic M, Varsani A, Wolf YI, Yutin N, et al. (May 2020). "Global Organization and Proposed Megataxonomy of the Virus World". Microbiology and Molecular Biology Reviews. 84 (2): e00061–19, /mmbr/84/2/MMBR.00061–19.atom. doi:10.1128/MMBR.00061-19. PMC 7062200. PMID 32132243.
- ^ Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Adriaenssens EM, Dempsey DM, et al. (November 2020). "Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses (2020)". Archives of Virology. 165 (11): 2737–2748. doi:10.1007/s00705-020-04752-x. PMID 32816125. S2CID 221182789.
- ^ Yutin N, Bäckström D, Ettema TJ, Krupovic M, Koonin EV (April 2018). "Vast diversity of prokaryotic virus genomes encoding double jelly-roll major capsid proteins uncovered by genomic and metagenomic sequence analysis". Virology Journal. 15 (1): 67. doi:10.1186/s12985-018-0974-y. PMC 5894146. PMID 29636073.
- ^ Bahar MW, Graham SC, Stuart DI, Grimes JM (July 2011). "Insights into the evolution of a complex virus from the crystal structure of vaccinia virus D13". Structure. 19 (7): 1011–20. doi:10.1016/j.str.2011.03.023. PMC 3136756. PMID 21742267.
- ^ Colson P, De Lamballerie X, Yutin N, Asgari S, Bigot Y, Bideshi DK, et al. (December 2013). ""Megavirales", a proposed new order for eukaryotic nucleocytoplasmic large DNA viruses". Archives of Virology. 158 (12): 2517–21. doi:10.1007/s00705-013-1768-6. PMC 4066373. PMID 23812617.
- ^ a b c d Krupovic, M; Makarova, KS; Koonin, EV (1 February 2022). "Cellular homologs of the double jelly-roll major capsid proteins clarify the origins of an ancient virus kingdom". Proceedings of the National Academy of Sciences of the United States of America. 119 (5): e2120620119. Bibcode:2022PNAS..11920620K. doi:10.1073/pnas.2120620119. PMC 8812541. PMID 35078938.
- ^ Butkovic, A; Dolja, VV; Koonin, EV; Krupovic, M (2023). "Plant virus movement proteins originated from jelly-roll capsid proteins". PLOS Biology. 21 (6): e3002157. doi:10.1371/journal.pbio.3002157. PMC 10306228. PMID 37319262.
- ^ Dutta S, Akey IV, Dingwall C, Hartman KL, Laue T, Nolte RT, et al. (October 2001). "The crystal structure of nucleoplasmin-core: implications for histone binding and nucleosome assembly". Molecular Cell. 8 (4): 841–53. doi:10.1016/S1097-2765(01)00354-9. PMID 11684019.
- ^ Edlich-Muth C, Artero JB, Callow P, Przewloka MR, Watson AA, Zhang W, et al. (May 2015). "The pentameric nucleoplasmin fold is present in Drosophila FKBP39 and a large number of chromatin-related proteins". Journal of Molecular Biology. 427 (10): 1949–63. doi:10.1016/j.jmb.2015.03.010. PMC 4414354. PMID 25813344.
- ^ Bodmer JL, Schneider P, Tschopp J (January 2002). "The molecular architecture of the TNF superfamily". Trends in Biochemical Sciences. 27 (1): 19–26. doi:10.1016/S0968-0004(01)01995-8. PMID 11796220.
- ^ Donadini R, Liew CW, Kwan AH, Mackay JP, Fields BA (January 2004). "Crystal and solution structures of a superantigen from Yersinia pseudotuberculosis reveal a jelly-roll fold". Structure. 12 (1): 145–56. doi:10.1016/j.str.2003.12.002. PMID 14725774.
- ^ Fraser JD, Proft T (October 2008). "The bacterial superantigen and superantigen-like proteins". Immunological Reviews. 225 (1): 226–43. doi:10.1111/j.1600-065X.2008.00681.x. PMID 18837785. S2CID 39174409.
- ^ Khuri S, Bakker FT, Dunwell JM (April 2001). "Phylogeny, function, and evolution of the cupins, a structurally conserved, functionally diverse superfamily of proteins". Molecular Biology and Evolution. 18 (4): 593–605. doi:10.1093/oxfordjournals.molbev.a003840. PMID 11264412.
- ^ Ozer A, Bruick RK (March 2007). "Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one?". Nature Chemical Biology. 3 (3): 144–53. doi:10.1038/nchembio863. PMID 17301803.
- ^ a b Aik W, McDonough MA, Thalhammer A, Chowdhury R, Schofield CJ (December 2012). "Role of the jelly-roll fold in substrate binding by 2-oxoglutarate oxygenases". Current Opinion in Structural Biology. 22 (6): 691–700. doi:10.1016/j.sbi.2012.10.001. PMID 23142576.
- ^ Chen Z, Zang J, Whetstine J, Hong X, Davrazou F, Kutateladze TG, et al. (May 2006). "Structural insights into histone demethylation by JMJD2 family members". Cell. 125 (4): 691–702. doi:10.1016/j.cell.2006.04.024. PMID 16677698. S2CID 15273763.
- ^ Klose RJ, Zhang Y (April 2007). "Regulation of histone methylation by demethylimination and demethylation". Nature Reviews. Molecular Cell Biology. 8 (4): 307–18. doi:10.1038/nrm2143. PMID 17342184. S2CID 2616900.
External links
[edit]- Antiparallel β Domains, a section from Anatomy and Taxonomy of Protein Structure by Jane S. Richardson
- The Jelly Roll of Life by Jacqueline Humphries at Small Things Considered, a blog sponsored by the American Society for Microbiology
