Curium(IV) oxide
Appearance
(Redirected from Curium dioxide)
| |
| Names | |
|---|---|
| Other names
Curium dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.453 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| CmO2 | |
| Molar mass | 279 g·mol−1 |
| Appearance | black crystals |
| insoluble | |
| Related compounds | |
Other cations
|
Americium(IV) oxide Berkelium(IV) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Curium(IV) oxide is an inorganic chemical compound of curium and oxygen with the chemical formula CmO2. Since all isotopes of curium are man-made, the compound does not occur in nature.
Synthesis
[edit]- Curium(IV) oxide can be prepared directly from the elements. Metallic curium is annealed in air or in an oxygen atmosphere:[1]
- Cm + O2 → CmO2
- Curium(III) hydroxide and curium(III) oxalate are also usually used for this purpose:
- Cm(OH)4 → CmO2 + 2H2O
- Cm(C2O4)2 → CmO2 + 2CO2 + 2CO
- Another way is the reaction of curium(III) oxide in an oxygen atmosphere at 650 °C:[2]
- 2Cm2O3 + O2 → 4CmO2
Physical properties
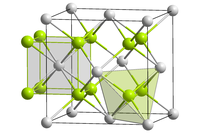
[edit]Curium(IV) oxide forms black crystals.[3] Insoluble in water. The compound crystals are of the cubic crystal system, the fluorite structure in the space group Fm3m.
Chemical properties
[edit]The compound reacts with mineral acids to form solutions of curium(III) salts.[4]
Uses
[edit]The compound is used for the manufacturing of isotopic current sources, also as targets for the synthesis of transcurium elements.
References
[edit]- ^ Asprey, L. B.; Ellinger, F. H.; Fried, S.; Zachariasen, W. H. (March 1955). "EVIDENCE FOR QUADRIVALENT CURIUM: X-RAY DATA ON CURIUM OXIDES1". Journal of the American Chemical Society. 77 (6): 1707–1708. doi:10.1021/ja01611a108. ISSN 0002-7863. Retrieved 29 June 2023.
- ^ Noé, M.; Fuger, J. (1 May 1971). "Self-radiation effects on the lattice parameter of 244CmO2". Inorganic and Nuclear Chemistry Letters. 7 (5): 421–430. doi:10.1016/0020-1650(71)80177-0. ISSN 0020-1650. Retrieved 29 June 2023.
- ^ Konings, R. J. M. (1 October 2001). "Thermochemical and thermophysical properties of curium and its oxides". Journal of Nuclear Materials. 298 (3): 255–268. Bibcode:2001JNuM..298..255K. doi:10.1016/S0022-3115(01)00652-3. ISSN 0022-3115. Retrieved 29 June 2023.
- ^ Lumetta, Gregg J.; Thompson, Major C.; Penneman, Robert A.; Eller, P. Gary (2006). "Curium". The Chemistry of the Actinide and Transactinide Elements. Springer Netherlands: 1397–1443. doi:10.1007/1-4020-3598-5_9. ISBN 978-1-4020-3555-5. Retrieved 29 June 2023.
