Transition metal carbonate and bicarbonate complexes
Transition metal carbonate and bicarbonate complexes are coordination compounds containing carbonate (CO32-) and bicarbonate (HCO3-) as ligands. The inventory of complexes is large, enhanced by the fact that the carbonate ligand can bind metal ions in a variety of bonding modes.[1][2] They illustrate the fate of low valent complexes when exposed to air.
Bonding modes
[edit]Carbonate
[edit]


Carbonate is a pseudohalide ligand. With a saturated pi-system, it has no pi-acceptor properties. With multiple electronegative elements, it is not strongly basic. The latter is consistent with the pKa’s of carbonic acid: pK1 = 6.77 and pK2 = 9.93.
To a single metal ion, carbonate is observed to bind in both unidentate (κ1-) and bidentate (κ2-) fashions.[5] In the covalent bond classification method, κ1-carbonate is anX ligand and κ2-carbonate is an X2 ligand. With two metals, the number of bonding modes increases because carbonate often serves as a bridging ligand. It can span metal-metal bonds as in [Ru2(CO3)4Cl2]5-, where again it functions as an (X)2 ligand. More commonly all three oxygen centers bind, as illustrated by [(C5H5)2Ti]2CO3. In such cases, carbonate is an LX ligand, providing 3e- to each metal. More complicated motifs have been characterized by X-ray crystallography including {(VO)6(μ-OH)9(CO3)4}5-.
Bicarbonate
[edit]
The bonding modes of bicarbonate are more limited than those for carbonate, in part because it is less basic and in part because the proton occupies a metal-binding site. Typically bicarbonate is assumed to bind as an unidentate X ligand. Structural studies on such complexes are, however, rare.[6]
Synthesis
[edit]Carbonato complexes are prepared by salt metathesis reactions using alkali metal carbonate salts as precursors. In some cases, bicarbonate intermediates are implicated since carbonate does not exist in appreciable concentrations near neutral pH. The other chief route to metal carbonato complexes involves addition of CO2 to metal oxides. Such reactions may be catalyzed by water since the carbonation of metal hydroxides is particularly well established. Isotope labeling studies show that these reactions can proceed (and perhaps usually proceed) without scission of the M-OH bond (L = generic ligand):
- [LnM−17OH]z + CO2 → [LnM−17OCO2H]z
Many esoteric routes have been demonstrated. For example, the deoxygenation of peroxycarbonate by tertiary phosphines:
- Pt(PPh3)2(O3CO) + PPh3 → Pt(PPh3)2(O2CO) + OPPh3 (Ph = C6H5)
Carbon dioxide undergoes disproportionation upon reaction with low-valence metals.[7]
Reactions
[edit]Most fundamental reactivity of bicarbonate/carbonato complexes is their interconversion. This acid-base reaction has been examined mainly for unimolecular complexes. Such reactions are molecular versions of the familiar reaction of acids with carbonate minerals.
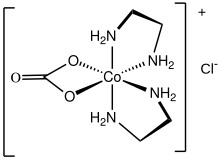
Protonation of carbonato complexes gives the corresponding bicarbonate. The structure of bicarbonate complex indicates that protonation occurs at the coordinated oxygen.[8] This process is the microscopic reverse or the first step in the carbonation of metal hydroxides. Protonation of bicarbonate ligands results in loss of carbon dioxide and formation of the metal hydroxide. Particularly well studied are the reactions of [Co(NH3)4(CO3)]+ and its ethylenediamine analogue carbonatobis(ethylenediamine)cobalt(III).[1]
Homoleptic complexes
[edit]Few homoleptic carbonato complexes have been characterized. One is [Zr(CO3)4]4-, featuring 8-ccordinate Zr(IV).[9] Tris(carbonato)cobalt(III) ([Co(CO)3]3-) is another example.
Use and natural occurrence
[edit]Metal carbonato and bicarbonate complexes are of no direct commercial importance. Several minerals are metal carbonates, and a few feature molecular carbonate complexes, e.g. hellyerite ([Ni2(CO3)2(H2O)8].H2O.[10]
In the biological sphere, zinc bicarbonate complexes are central intermediates in the action of the carbonic anhydrase:[11]
- [(imidazole)3ZnOH]+ + CO2 ⇌ [(imidazole)3ZnOCO2H]+
References
[edit]- ^ a b Krishnamurty, Kotra V.; Mc Leod Harris, Gordon.; Sastri, Vedula S. (1970). "Chemistry of the Metal Carbonato Complexes". Chemical Reviews. 70 (2): 171–197. doi:10.1021/cr60264a001.
- ^ Palmer, Donald A.; Van Eldik, Rudi (1983). "The Chemistry of Metal Carbonato and Carbon Dioxide Complexes". Chemical Reviews. 83 (6): 651–731. doi:10.1021/cr00058a004.
- ^ Spannenberg, A.; Zippel, Τ.; Burlakov, V. V.; Rosenthal, U. (2000). "Crystal Structure of Di(bis(cyclopentadienyl)titanocene) Carbonate, C42H40O6Ti4". Zeitschrift für Kristallographie - New Crystal Structures. 215 (3): 367–368. doi:10.1515/ncrs-2000-0330. S2CID 97915556.
- ^ Yang, Jian-Hui; Cheng, Ru-Mei; Jia, Yan-Yan; Jin, Jin; Yang, Bing-Bing; Cao, Zhi; Liu, Bin (2016). "Chlorine and Temperature Directed Self-Assembly of Mg–Ru2(II,III) Carbonates and Particle Size Dependent Magnetic Properties". Dalton Transactions. 45 (7): 2945–2954. doi:10.1039/C5DT04463D. PMID 26750871.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Crutchley, R. J.; Powell, J.; Faggiani, R.; Lock, C. J. L. (1977). "The Formation and Molecular Structure of a Monodentate Bicarbonate Complex of Palladium(II)". Inorganica Chimica Acta. 24: L15–L16. doi:10.1016/S0020-1693(00)93807-6.
- ^ Gibson, Dorothy H. (1996). "The Organometallic Chemistry of Carbon Dioxide". Chemical Reviews. 96 (6): 2063–2096. doi:10.1021/cr940212c. PMID 11848822.
- ^ Romero, Antonio; Santos, Amelia; Vegas, Angel (1988). "Reactions of [Ru(CO)CLH(Me2Hpz)(PR3)2] (Me2Hpz = 3,5-dimethylpyrazole; R = Ph, p-tolyl) with Acetylenes. The Crystal Structure of [Ru(CO)Cl(HC:CHCMe3)(Me2HPz)(PPh3)2] and [Ru(CO)(MeO2CC:CHCO2Me)(HCO3)(PPh3)2]". Organometallics. 7 (9): 1988–1993. doi:10.1021/om00099a014.
- ^ Yu E. Gorbunova; V. G. Kuznetsov; E. S. Kovaleva (1968). "Russian Journal of Inorganic Chemistry". Zh. Neorg. Khim. (Russ. J. Inorg. Chem.). 13: 51.
- ^ Bette, Sebastian; Rincke, Christine; Dinnebier, Robert E.; Voigt, Wolfgang (2016). "Crystal Structure and Hydrate Water Content of Synthetic Hellyerite, NiCO3·5.5H2O". Zeitschrift für Anorganische und Allgemeine Chemie. 642 (9–10): 652–659. doi:10.1002/zaac.201600044.
- ^ Sattler, Wesley; Parkin, Gerard (2012). "Structural Characterization of Zinc Bicarbonate Compounds Relevant to the Mechanism of Action of Carbonic Anhydrase". Chemical Science. 3 (6): 2015. doi:10.1039/c2sc20167d.
