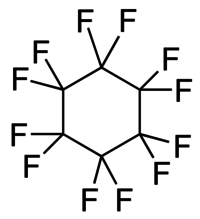
Perfluorocyclohexane
Appearance
(Redirected from C6F12)
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dodecafluorocyclohexane | |
| Other names
1,1,2,2,3,3,4,4,5,5,6,6-Dodecafluorocyclohexane, Cyclohexane, dodecafluoro-
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.005.993 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6F12 | |
| Molar mass | 300.047 g·mol−1 |
| Appearance | clear, waxy solid |
| Density | 1.684 g/cm3 |
| Melting point | 52 °C (126 °F; 325 K)[1] |
| Boiling point | 59–60 °C (138–140 °F; 332–333 K) |
| Solubility | Miscible with organic compounds |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Safety data sheet (SDS) | MSDS [1] |
| Related compounds | |
Related compounds
|
Fluorocarbon |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Perfluorocyclohexane or dodecafluorocyclohexane is a chemical which belongs to the class of fluorocarbons, sometimes referred to as perfluorocarbons or PFCs. Fluorocarbons and their derivatives are useful fluoropolymers, refrigerants, solvents, and anesthetics.
Synthesis
[edit]Perfluorocyclohexane can be synthesized by fluorination of cyclohexane.[2]
Properties
[edit]Perfluorocyclohexane is chemically inert and thermally stable. It is a relatively non-toxic, clear, waxy solid, which has a high vapor pressure and therefore sublimes readily at room temperature.[citation needed]
The molecule predominantly exists in its chair conformation, in which it possesses D3d molecular symmetry.
References
[edit]- ^ Sander, M.; Blöchl, W. (January 1965). "Herstellung von Perfluoralkanen und Perfluorcycloalkanen". Chemie Ingenieur Technik (in German). 37 (1): 7–13. doi:10.1002/cite.330370103.
- ^ Sandford G. (2003). "Perfluoroalkanes". Tetrahedron. 59 (4): 437–454. doi:10.1016/s0040-4020(02)01568-5.
