Lilial

| |
| Names | |
|---|---|
| IUPAC name
3-(4-tert-Butylphenyl)-2-methylpropanal
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.173 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 3082 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H20O | |
| Molar mass | 204.313 g·mol−1 |
| Appearance | Clear viscous liquid |
| Density | 0.94 g/cm3 |
| Melting point | −20 °C (−4 °F; 253 K) |
| Boiling point | 275 °C (527 °F; 548 K)[1] |
| 0.045 g/L at 20 °C | |
| log P | 4.36 [1] |
| Pharmacology | |
| Topical | |
| Related compounds | |
Related aldehydes
|
Bourgeonal |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lilial (a trade name for lily aldehyde, also known as lysmeral or lilestralis) is a chemical compound commonly used as a perfume in cosmetic preparations and laundry powders, often under the name butylphenyl methylpropional. It is an aromatic aldehyde, naturally occurring in crow-dipper and tomato plants,[2] and produced synthetically in large scale. It was banned for use in cosmetics by the EU in March 2022 after being found to be harmful to fertility.
Synthesis
[edit]Lilial is produced by the mixed aldol coupling of 4-tert-butylbenzaldehyde (typically as it's methanol acetal) and propionaldehyde, followed by hydrogenation.
Properties
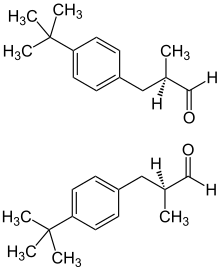
[edit]Lilial is commonly produced and sold as a racemic mixture; however, testing has indicated that the different enantiomers of the compound do not contribute equally to its odor. The (R)-enantiomer has a strong floral odor, reminiscent of cyclamen or lily of the valley; whereas the (S)-enantiomer possesses no strong odor.[3]
Like most aldehydes, lilial is not long term stable and tends to slowly oxidize on storage.
Safety
[edit]The Scientific Committee on Consumer Safety (SCCS, scientific committee for consumer safety of the EU Commission) concluded in May 2019 that the use of lilial in both rinse-off and leave-on cosmetics "cannot be considered as safe".[4]
After animal studies found it to be toxic for reproduction, it was reclassified as a prohibited substance in the EU, and banned from use in cosmetics as of March 2022.[5]
It can sometimes act as an allergen and may cause contact dermatitis in susceptible individuals.
See also
[edit]References
[edit]- ^ a b Haefliger, Olivier P.; Jeckelmann, Nicolas; Ouali, Lahoussine; León, Géraldine (2010). "Real-Time Monitoring of Fragrance Release from Cotton Towels by Low Thermal Mass Gas Chromatography Using a Longitudinally Modulating Cryogenic System for Headspace Sampling and Injection". Analytical Chemistry. 82 (2): 729–737. doi:10.1021/ac902460d. ISSN 0003-2700. PMID 20025230.
- ^ "Lilial".
- ^ Bartschat, Dietmar; Bürner, Susanne; Mosandl, A.; Bats, Jan W. (1997). "Stereoisomeric flavour compounds LXXVI: direct enantioseparation, structure elucidation and structure-function relationship of 4-tert-butyl-α-methyldihydrocinnamaldehyde". Zeitschrift für Lebensmittel-Untersuchung und -Forschung A. 205 (1): 76–79. doi:10.1007/s002170050127. ISSN 1431-4649. S2CID 97399242.
- ^
Scientific Committee on Consumer Safety (2019-05-10). "OPINION ON the safety of Butylphenyl methylpropional (p-BMHCA) in cosmetic products" (PDF). Retrieved 2020-05-28.
On individual product basis, Butylphenyl methylpropional (p-BMHCA) (CAS 80-54-6) with alpha-tocopherol at 200 ppm, can be considered safe when used as fragrance ingredient in different cosmetic leave-on and rinse-off type products. However, considering the first-tier deterministic aggregate exposure, arising from the use of different product types together, Butylphenyl methylpropional at the proposed concentrations cannot be considered as safe.
- ^ "COMMISSION REGULATION (EU) 2021/1902 OF 29 October 2021 amending Annexes II, III and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council as regards the use of cosmetic products of certain substances classified as carcinogenic, mutagenic or toxic for reproduction". Retrieved 2022-01-03.
CAS No. 80-54-6