Blood donation



A blood donation occurs when a person voluntarily has blood drawn and used for transfusions and/or made into biopharmaceutical medications by a process called fractionation (separation of whole blood components). A donation may be of whole blood, or of specific components directly (apheresis). Blood banks often participate in the collection process as well as the procedures that follow it.
Today in the developed world, most blood donors are unpaid volunteers who donate blood for a community supply. In some countries, established supplies are limited and donors usually give blood when family or friends need a transfusion (directed donation). Many donors donate for several reasons, such as a form of charity, general awareness regarding the demand for blood, increased confidence in oneself, helping a personal friend or relative, and social pressure. Despite the many reasons that people donate, not enough potential donors actively donate. However, this is reversed during disasters when blood donations increase, often creating an excess supply that will have to be later discarded. In countries that allow paid donation some people are paid, and in some cases there are incentives other than money such as paid time off from work. People can also have blood drawn for their own future use (autologous donation). Donating is relatively safe, but some donors have bruising where the needle is inserted or may feel faint.
Potential donors are evaluated for anything that might make their blood unsafe to use. The screening includes testing for diseases that can be transmitted by a blood transfusion, including HIV and viral hepatitis. The donor must also answer questions about medical history and take a short physical examination to make sure the donation is not hazardous to their health. How often a donor can donate varies from days to months based on what component they donate and the laws of the country where the donation takes place. For example, in the United States, donors must wait 56 days (eight weeks) between whole-blood donations but only seven days between platelet apheresis donations and twice per seven-day period in plasmapheresis.[1]: How often can I donate blood?
The amount of blood drawn and the methods vary. The collection can be done manually or with automated equipment that takes only specific components of the blood. Most of the components of blood used for transfusions have a short shelf life, and maintaining a constant supply is a persistent problem. This has led to some increased interest in autotransfusion, whereby a patient's blood is salvaged during surgery for continuous reinfusion—or alternatively, is self-donated prior to when it will be needed. Generally, the notion of donation does not refer to giving to one's self, though in this context it has become somewhat acceptably idiomatic.
History
[edit]Charles Richard Drew (1904–1950) was an American surgeon and medical researcher. His research was in the field of blood transfusions, developing improved techniques for blood storage, and applied his expert knowledge to developing large-scale blood banks early in World War II. This allowed medics to save thousands of lives of the Allied forces. As the most prominent African American in the field, Drew protested against the practice of racial segregation in the donation of blood, as it lacked scientific foundation, and resigned his position with the American Red Cross, which maintained the policy until 1950.[citation needed]
Types of donation
[edit]
Blood donations are divided into groups based on who will receive the collected blood.[2] An allogeneic (also called homologous) donation is when a donor gives blood for storage at a blood bank for transfusion to an unknown recipient. A directed donation is when a person, often a family member, donates blood for transfusion to a specific individual.[3] Directed donations are relatively rare when an established supply exists.[4] A replacement donor donation is a hybrid of the two and is common in developing countries.[5] In this case, a friend or family member of the recipient donates blood to replace the stored blood used in a transfusion, ensuring a consistent supply. When a person has blood stored that will be transfused back to the donor at a later date, usually after surgery, that is called an autologous donation.[6] Blood that is used to make medications can be made from allogeneic donations or from donations exclusively used for manufacturing.[7]
Sometimes there are specific reasons for preferring one form or the other. Allogeneic donations have a lower risk of some complications than blood from a family member.[8] Neonatal alloimmune thrombocytopenia may require a transfusion from the mother's own platelets.[8] Autologous (self) donations may be preferred for someone with a rare blood type for a planned surgery.[8]
Blood is sometimes collected using similar methods for therapeutic phlebotomy, similar to the ancient practice of bloodletting, which is used to treat conditions such as hereditary hemochromatosis or polycythemia vera. This blood is sometimes treated as a blood donation, but may be immediately discarded if it cannot be used for transfusion or further manufacturing.[9]
The actual process varies according to the laws of the country, and recommendations to donors vary according to the collecting organization.[10][11][12] The World Health Organization gives recommendations for blood donation policies,[13] but in developing countries many of these are not followed. For example, the recommended testing requires laboratory facilities, trained staff, and specialized reagents, all of which may not be available or too expensive in developing countries.[14]

A blood drive or a blood donor session is an event in which donors come to donate allogeneic blood. These can occur at a blood bank, but they are often set up at a location in the community, such as a shopping center, workplace, school, or house of worship.[15]
Screening
[edit]Donors are typically required to give consent for the process, and meet a certain criteria such as weight and hemoglobin levels, and this requirement means minors cannot donate without permission from a parent or guardian.[16] In some countries, answers are associated with the donor's blood, but not name, to provide anonymity; in others, such as the United States, names are kept to create lists of ineligible donors.[17] If a potential donor does not meet these criteria, they are 'deferred'. This term is used because many donors who are ineligible may be allowed to donate later. Blood banks in the United States may be required to label the blood if it is from a therapeutic donor, so some do not accept donations from donors with any blood disease.[18] Others, such as the Australian Red Cross Blood Service, accept blood from donors with hemochromatosis. It is a genetic disorder that does not affect the safety of the blood.[19]
The donor's race or ethnic background is sometimes important since certain blood types, especially rare ones, are more common in certain ethnic groups.[20] Historically, in the United States donors were segregated or excluded on race, religion, or ethnicity, but this is no longer a standard practice.[21][22]
Recipient safety
[edit]

Donors are screened for health risks that could make the donation unsafe for the recipient. Some of these restrictions are controversial, such as restricting donations from men who have sex with men (MSM) because of the risk of transmitting HIV.[23] In 2011, the UK (excluding Northern Ireland) reduced its blanket ban on MSM donors to a narrower restriction which only prevents MSM from donating blood if they have had sex with other men within the past year.[24] A similar change was made in the US in late 2015 by the Food and Drug Administration (FDA).[25] In 2017, the UK and US further reduced their restrictions to three months.[26] In 2023, the FDA announced new policies easing restrictions on gay and bisexual men donating blood.[27] These updated guidelines stipulate that men in monogamous relationships with other men, or who have not recently had sex, can donate.[27] Individuals who report having sex with people who are HIV positive or have had sex with a new partner who has engaged in anal sex are still barred from blood donation.[27] Autologous donors are not always screened for recipient safety problems since the donor is the only person who will receive the blood.[28] Since the donated blood may be given to pregnant women or women of child-bearing age, donors taking teratogenic (birth defect-causing) medications are deferred. These medications include acitretin, etretinate, isotretinoin, finasteride, and dutasteride.[29]
Donors are examined for signs and symptoms of diseases that can be transmitted in a blood transfusion, such as HIV, malaria, and viral hepatitis. Screening may include questions about risk factors for various diseases, such as travel to countries at risk for malaria or variant Creutzfeldt–Jakob disease (vCJD). These questions vary from country to country. For example, while blood centers in Québec and the rest of Canada, Poland, and many other places defer donors who lived in the United Kingdom for risk of vCJD,[30][31] donors in the United Kingdom are only restricted for vCJD risk if they have had a blood transfusion in the United Kingdom.[32] Australia removed its UK-donor deferral in July 2022.[33]
Directed donations from family members (e.g., a father donating blood to his child) carry extra risks for the recipient.[8] Any blood transfusion carries some risk of a transfusion reaction, but between genetically related family members, there are additional risks.[8] The donated blood must be irradiated to prevent a potentially deadly graft-versus-host disease, which is more likely between genetically related people.[8] Not all healthcare facilities have the equipment to do this on site.[8] Alloimmunization is a particular risk for directed granulocyte donations.[8] It is a common misconception that directed donations are safer for the recipient; however, family members and close friends, especially parents who have not previously donated blood, frequently feel pressured into lying about disqualifying risk factors (e.g., drug use or prior sexual relationships) and their eligibility, which can result in a higher risk of infection with bloodborne pathogens.[8] Additionally, in the less common case of a person with leukemia or other bone marrow disease, a familial blood transfusion can trigger the production of alloantibodies against HLA proteins, which can cause a bone marrow transplant from that donor to fail in the future.[8] (Closely related family members are usually the best match for a bone marrow transplant.[8]) A directed donation from an unrelated friend, however, would not have the same risk.
Donor safety
[edit]The donor is also examined and asked specific questions about their medical history to make sure that donating blood is not hazardous to their health. The donor's hematocrit or hemoglobin level is tested to make sure that the loss of blood will not make them anemic, and this check is the most common reason that a donor is ineligible.[34] Accepted hemoglobin levels for blood donations, by the American Red Cross, is 12.5g/dL (for females) and 13.0g/dL (for males) to 20.0g/dL, anyone with a higher or lower hemoglobin level cannot donate.[35] Pulse, blood pressure, and body temperature are also evaluated. Elderly donors are sometimes also deferred on age alone because of health concerns.[36] In addition to age, weight and height are important factors when considering the eligibility for donors. For example, the American Red Cross requires a donor to be 110 pounds (50 kg) or more for whole blood and platelet donation and at least 130 pounds (59 kg) (males) and at least 150 pounds (68 kg) (females) for power red donations (double red erythrocytapheresis).[37] The safety of donating blood during pregnancy has not been studied thoroughly, and pregnant women are usually deferred until six weeks after the pregnancy.[38] Donors with aortic stenosis have traditionally been deferred out of concern that the acute volume depletion (475 mL) of blood donation might compromise cardiac output.[39]
Blood testing
[edit]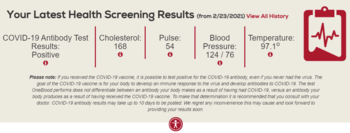
The donor's blood type must be determined if the blood will be used for transfusions. The collecting agency usually identifies whether the blood is type A, B, AB, or O and the donor's Rh (D) type and will screen for antibodies to less common antigens. More testing, including a crossmatch, is usually done before a transfusion. Type O negative is often cited as the "universal donor"[40] but this only refers to red cell and whole blood transfusions. For plasma and platelet transfusions the system is reversed: AB positive is the universal platelet donor type while both AB positive and AB negative are universal plasma donor types.[41][42]
Most blood is tested for diseases, including some STDs.[43] The tests used are high-sensitivity screening tests and no actual diagnosis is made. Some of the test results are later found to be false positives using more specific testing.[44] False negatives are rare, but donors are discouraged from using blood donation for the purpose of anonymous STD screening because a false negative could mean a contaminated unit. The blood is usually discarded if these tests are positive, but there are some exceptions, such as autologous donations. The donor is generally notified of the test result.[45]
Donated blood is tested by many methods, but the core tests recommended by the World Health Organization are these four:[46]
- Hepatitis B surface antigen
- Antibody to hepatitis C
- Antibody to HIV, usually subtypes 1 and 2
- Serologic test for syphilis
The WHO reported in 2006 that 56 out of 124 countries surveyed did not use these basic tests on all blood donations.[14]
A variety of other tests for transfusion transmitted infections are often used based on local requirements. Additional testing is expensive, and in some cases the tests are not implemented because of the cost.[47] These additional tests include other infectious diseases such as West Nile fever[48] and babesiosis.[49] Sometimes multiple tests are used for a single disease to cover the limitations of each test. For example, the HIV antibody test will not detect a recently infected donor, so some blood banks use a p24 antigen or HIV nucleic acid test in addition to the basic antibody test to detect infected donors. Cytomegalovirus is a special case in donor testing in that many donors will test positive for it.[50] The virus is not a hazard to a healthy recipient, but it can harm infants[51] and other recipients with weak immune systems.[50]
Blood testing in the US takes at least 48 hours.[52] Because of the time required for testing, directed donations are not practical in emergencies.[8][52]
Obtaining the blood
[edit]

There are two main methods of obtaining blood from a donor. The most frequent is to simply take the blood from a vein as whole blood. This blood is typically separated into parts, usually red blood cells and plasma, since most recipients (other than trauma patients) need only a specific component for transfusions.[53]
The amount of blood donated in one session – generally called a 'unit' – is defined by the WHO as 450 millilitres.[54] Some countries like Canada follow this standard,[55] but others have set their own rules, and sometimes there is variation even among different agencies within a country. For example, whole blood donations in the United States are in the 460–500 ml range,[56][57] while those in the EU must be in the 400-500 ml range.[58] Other countries have smaller units – India uses 350 ml,[59] Singapore 350 or 450 ml,[60] and Japan 200 or 400 ml.[61] Historically, donors in the People's Republic of China would donate only 200 ml, though larger 300 and 400 ml donations have become more common, particularly in northern China and for heavier donors.[62] In any case, an additional 5-10 ml of blood may be collected separately for testing.
The other method is to draw blood from the donor, separate it using a centrifuge or a filter, store the desired part, and return the rest to the donor. This process is called apheresis, and it is often done with a machine specifically designed for this purpose. This process is especially common for plasma, platelets, and red blood cells.[63]
For direct transfusions a vein can be used but the blood may be taken from an artery instead.[64] In this case, the blood is not stored, but is pumped directly from the donor into the recipient. This was an early method for blood transfusion and is rarely used in modern practice.[65] It was phased out during World War II because of problems with logistics, and doctors returning from treating wounded soldiers set up banks for stored blood when they returned to civilian life.[66]
Site preparation and drawing blood
[edit]The blood is drawn from a large arm vein close to the skin, usually the median cubital vein on the inside of the elbow. The skin over the blood vessel is cleaned with an antiseptic such as iodine or chlorhexidine[67] to prevent skin bacteria from contaminating the collected blood[67] and also to prevent infections where the needle pierced the donor's skin.[68]
A large[69] needle (16 to 17 gauge) is used to minimize shearing forces that may physically damage red blood cells as they flow through the needle.[70] A tourniquet is sometimes wrapped around the upper arm to increase the pressure of the blood in the arm veins and speed up the process. The donor may also be prompted to hold an object and squeeze it repeatedly to increase the blood flow through the vein.[citation needed]

Whole blood
[edit]The most common method is collecting the blood from the donor's vein into a container. The amount of blood drawn varies from 200 millilitres to 550 millilitres depending on the country, but 450 millilitres is typical.[54] The blood is usually stored in a flexible plastic bag that also contains sodium citrate, phosphate, dextrose, and adenine. This combination keeps the blood from clotting and preserves it during storage up to 42 days.[71][72][73] Other chemicals are sometimes added during processing.
The plasma from whole blood can be used to make plasma for transfusions or it can also be processed into other medications using a process called fractionation. This was a development of the dried plasma used to treat the wounded during World War II and variants on the process are still used to make a variety of other medications.[74][75]
Apheresis
[edit]
Apheresis is a blood donation method where the blood is passed through an apparatus that separates out one particular constituent and returns the remainder to the donor. Usually the component returned is the red blood cells, the portion of the blood that takes the longest to replace. Using this method an individual can donate plasma or platelets much more frequently than they can safely donate whole blood.[76] These can be combined, with a donor giving both plasma and platelets in the same donation.[citation needed]
Platelets can also be separated from whole blood, but they must be pooled from multiple donations. From three to ten units of whole blood are required for a therapeutic dose.[77] Plateletpheresis provides at least one full dose from each donation.[citation needed]
During a platelet donation, the blood is drawn from the patient and the platelets are separated from the other blood components. The remainder of the blood, red blood cells, plasma, and white blood cells are returned to the patient. This process is completed several times for a period of up to two hours to collect a single donation.[78]
Plasmapheresis is frequently used to collect source plasma that is used for manufacturing into medications much like the plasma from whole blood. Plasma collected at the same time as plateletpheresis is sometimes called concurrent plasma.
Apheresis is also used to collect more red blood cells than usual in a single donation (commonly known as "double reds") and to collect white blood cells for transfusion.[79][80]

Recovery and time between donations
[edit]Donors are usually kept at the donation site for 10–15 minutes after donating since most adverse reactions take place during or immediately after the donation.[81] Blood centers typically provide light refreshments, such as orange juice and cookies, or a lunch allowance to help the donor recover.[82] The needle site is covered with a bandage and the donor is directed to keep the bandage on for several hours.[56] In hot climates, donors are advised to avoid dehydration (strenuous exercise and games, alcohol) until a few hours after donation.[citation needed]
Donated plasma is replaced after 2–3 days.[83] Red blood cells are replaced by bone marrow into the circulatory system at a slower rate, on average 36 days in healthy adult males. In one study, the range was 20 to 59 days for recovery.[84] These replacement rates are the basis of how frequently a donor can donate blood.[citation needed]
Plasmapheresis and plateletpheresis donors can donate much more frequently because they do not lose significant amounts of red cells. The exact rate of how often a donor can donate differs from country to country. For example, plasmapheresis donors in the United States are allowed to donate large volumes twice a week and could nominally donate 83 litres (about 22 gallons) in a year, whereas the same donor in Japan may only donate every other week and could only donate about 16 litres (about 4 gallons) in a year.[85]
Iron supplementation decreases the rates of donor deferral due to low hemoglobin, both at the first donation visit and at subsequent donations. Iron-supplemented donors have higher hemoglobin and iron stores. On the other hand, iron supplementation frequently causes diarrhea, constipation and epigastric abdominal discomfort. The long-term effects of iron supplementation without measurement of iron stores are unknown.[86]
Complications
[edit]Donors are screened for health problems that would put them at risk for serious complications from donating. First-time donors, teenagers, and women are at a higher risk of a reaction.[87][88] One study showed that 2% of donors had an adverse reaction to donation.[89] Most of these reactions are minor. A study of 194,000 donations found only one donor with long-term complications.[90] In the United States, a blood bank is required to report any death that might possibly be linked to a blood donation. An analysis of all reports from October 2008 to September 2009 evaluated six events and found that five of the deaths were clearly unrelated to donation, and in the remaining case they found no evidence that the donation was the cause of death.[91]

Hypovolemic reactions can occur because of a rapid change in blood pressure. Fainting is generally the worst problem encountered.[92] Falls due to loss of consciousness may result in injuries in rare cases. [93] The risk for developing dizziness with fainting is increased in female and young donors. [94]
The process has similar risks to other forms of phlebotomy. Bruising of the arm from the needle insertion is the most common concern. One study found that less than 1% of donors had this problem.[95] A number of less common complications of blood donation are known to occur. These include arterial puncture, delayed bleeding, nerve irritation, nerve injury, tendon injury, thrombophlebitis, and allergic reactions.[96]
Donors sometimes have adverse reactions to the sodium citrate used in apheresis collection procedures to keep the blood from clotting. Since the anticoagulant is returned to the donor along with blood components that are not being collected, it can bind the calcium in the donor's blood and cause hypocalcemia.[97] These reactions tend to cause tingling in the lips, but may cause convulsions, seizure, hypertension, or more serious problems.[98] Donors are sometimes given calcium supplements during the donation to prevent these side effects.[99]
In apheresis procedures, the red blood cells are returned. If this is done manually and the donor receives the blood from a different donor, a transfusion reaction can take place. Manual apheresis is extremely rare in the developed world because of this risk and automated procedures are as safe as whole blood donations.[100]
The final risk to blood donors is from equipment that has not been properly sterilized.[101] In most cases, the equipment that comes in direct contact with blood is discarded after use.[102] Re-used equipment was a significant problem in China in the 1990s, and up to 250,000 blood plasma donors may have been exposed to HIV from shared equipment.[103][104][105]
Storage, supply and demand
[edit]
Storage and blood shelf life
[edit]The collected blood is usually stored in a blood bank as separate components, and some of these have short shelf lives. There are no storage methods to keep platelets for extended periods of time, though some were being studied as of 2008.[106] The longest shelf life used for platelets is seven days.[107]
Red blood cells (RBC), the most frequently used component, have a shelf life of 35–42 days at refrigerated temperatures.[108][109] For (relatively rare) long-term storage applications, this can be extended by freezing the blood with a mixture of glycerol, but this process is expensive and requires an extremely cold freezer for storage.[50] Plasma can be stored frozen for an extended period of time and is typically given an expiration date of one year and maintaining a supply is less of a problem.[110]
Demand for blood
[edit]The examples and perspective in this section deal primarily with the United States and do not represent a worldwide view of the subject. (August 2024) |
The American Red Cross states that in the United States, someone needs blood every two seconds, and someone needs platelets every thirty seconds.[111] There is not a consistent demand for each blood type. One type of blood being in stock does not guarantee that another type is. Blood banks may have some units in stock but lack others, ultimately causing the patients that need units for specific blood types to have delayed or canceled procedures.[112] Additionally, every year there is an increase of around 5-7% for transfusions without an increase of donors to balance it as well as a growing population of elderly people that will need more transfusions in the future without a predicted increase in donations[113] to reflect those growing numbers. This was supported in 1998 where blood donations to the Red Cross increased to 8%, totaling 500,000 units but hospitals' need for donations increased by 11%.[114]
Blood donations tend to always be high in demand with numerous accounts repeatedly stating periodic shortages over the decades.[115] However, this trend is disrupted during national disasters. The trend demonstrates that people are donating the most during catastrophes when, arguably, donations are not as needed compared to periods without disasters.[115] From 1988 to 2013, it has been reported that during every national disaster, there was a surplus of donations; a surplus that consisted of over 100 units.[116] One of the most notable examples of this pattern was the September 11th attacks. A study observed that compared to the four weeks before September 11, there was an estimated increase of 18,700 donations from first-time donors for the first week after the attack: 4,000 was the average of donations from first-time donors before the attack which increased to about 22,700 donations; while repeat donors increased their donations by 10,000 per week: initially, donations were estimated to be around 16,400 which increased to 26,400 donations after September 11.[117] Therefore, in the first week after the attack on 9/11, there was an overall estimated 28,700 increase in donations compared to the average weekly donations made four weeks prior to the attack. Increases in donations were observed in all blood donation centers, beginning on the day of the attack.[117] While blood donations were above average after the first few weeks following 9/11, the number of donations fell from an estimated 49,000 donations in the first week to 26,000–28,000 donations between the second and fourth weeks after 9/11.[117][115] Despite the substantial increase of donors, the rate that first-time donors would become repeat donors were the same before and after the attack.[117]
The limited storage time means that it is difficult to have a stockpile of blood to prepare for a disaster. The subject was discussed at length after the September 11 attacks in the United States, and the consensus was that collecting during a disaster was impractical and that efforts should be focused on maintaining an adequate supply at all times.[118] Blood centers in the U.S. often have difficulty maintaining even a three-day supply for routine transfusion demands.[119]
Donation levels
[edit]The World Health Organization (WHO) recognizes World Blood Donor Day on 14 June each year to promote blood donation. This is the birthday of Karl Landsteiner, the scientist who discovered the ABO blood group system.[120] The theme of the 2012 World Blood Donor Day campaign, "Every blood donor is a hero" focuses on the idea that everyone can become a hero by giving blood. Based on data reported by 180 countries between 2011 and 2013, the WHO estimated that approximately 112.5 million units of blood were being collected annually.[121]
In the United States it is estimated that 111 million citizens are eligible blood donors,[122] or 37% of the population.[123] However less than 10% of the 37% eligible blood donors donate annually.[123] In the UK the NHS reports blood donation levels at "only 4%"[124] while in Canada the rate is 3.5%.[125]
Donator's incentive and deterrence
[edit]Multiple studies have shown that the main reason people donate is due to prosocial motivators (e.g., altruism, selflessness, charity), general awareness regarding the demand for blood, increased confidence in oneself, helping a personal friend/relative, and social pressure.[126][127] On the other hand, lack of blood donations can occur due to fear, lack of faith in the medical professionals, inconvenience, and the lack of consideration for donating,[128] or perceived racial discrimination.[127] Pathologist Leo McCarthy states that blood shortages routinely occur in the United States between July 4 and Labor day and between Christmas and New Year.[113]
Donor health benefits
[edit]In patients prone to iron overload, blood donation prevents the accumulation of toxic quantities.[129] Donating blood may reduce the risk of heart disease for men, but the link has not been firmly established and may be from selection bias because donors are screened for health problems.[130][131]
Research published in 2012 demonstrated that in patients with metabolic syndrome, repeated blood donation is effective in reducing blood pressure, blood glucose, HbA1c, low-density lipoprotein/high-density lipoprotein ratio, and heart rate.[132]
A study published in JAMA Network Open tracked PFAS levels in a clinical trial and showed that regular blood or plasma donations resulted in a significant reduction in PFAS levels for the participants.[133]
Donor compensation
[edit]


The World Health Organization set a goal in 1997 for all blood donations to come from unpaid volunteer donors, but as of 2006, only 49 of 124 countries surveyed had established this as a standard.[14] Some countries, such as Tanzania, have made great strides in moving towards this standard, with 20 percent of donors in 2005 being unpaid volunteers and 80 percent in 2007, but 68 of 124 countries surveyed by WHO had made little or no progress.[5] Most plasmapheresis donors in the United States are still paid for their donations.[134] Donors are now paid between $25 and $50 per donation.[135] In some countries, for example Brazil and the United Kingdom, it is illegal to receive any compensation, monetary or otherwise, for the donation of blood or other human tissues.[136]
Regular donors are often given some sort of non-monetary recognition. Time off from work is a common benefit.[137] For example, in Italy, blood donors receive the donation day as a paid holiday from work.[138] In 2023, Poland introduced legislation that secured two days off work for employed persons when they donate - the donation day and the subsequent next day.[139] Blood centers will also sometimes add incentives such as assurances that donors would have priority during shortages, free T-shirts, first aid kits, windshield scrapers, pens, and similar trinkets. There are also incentives for the people who recruit potential donors, such as prize drawings for donors and rewards for organizers of successful drives.[140] Recognition of dedicated donors is common. For example, the Singapore Red Cross Society presents awards for voluntary donors who have made a certain number of donations under the Blood Donor Recruitment Programme starting with a "bronze award" for 25 donations.[141] In Ireland the Irish Blood Transfusion Service awards a silver pin or pendant for 10 donations, a gold pin or pendant for 20 donations, a gold lapel pin for 50 donations while those reaching 100 donations attend a dinner ceremony where they are presented with a small porcelain statue depicting the logo of the IBTS (a pelican).[142] The government of Malaysia also offers free outpatient and hospitalization benefits for blood donors, for example, 4 months of free outpatient treatment and hospitalization benefits after every donation.[143] In Poland, after donating a specific amount of blood (18 litres for men and 15 for women), a person is gifted with the title of "Distinguished Honorary Blood Donor" as well as a medal. In addition, a popular privilege in larger Polish cities is the right to free use of public transport, but the conditions for obtaining a privilege may vary depending on the city. Also in Poland, Poznań's theatre Teatr Nowy offers theatregoers standing discounts on theatre tickets.[144] During the COVID-19 pandemic, many US blood centers advertised free COVID-19 antibody testing as an incentive to donate; however, these antibody tests were also useful for blood centers in determining which donors could be flagged for convalescent plasma donations.[145][146][147]
Most allogeneic blood donors donate as an act of charity and do not expect to receive any direct benefit from the donation.[148] The sociologist Richard Titmuss, in his 1970 book The Gift Relationship: From Human Blood to Social Policy, compared the merits of the commercial and non-commercial blood donation systems of the US and the UK, coming down in favor of the latter. The book became a bestseller in the US, resulting in legislation to regulate the private market in blood.[149] The book is still referenced in modern debates about turning blood into a commodity.[150] The book was republished in 1997 and the same ideas and principles are applied to analogous donation programs, such as organ donation and sperm donation.[151]
In low-resource countries, directed donation from family members and friends is common, because there is no other realistic option.[8] The practice causes ethical challenges, because the donor may feel coerced or may be receiving undisclosed payments.[8] Additionally, relying on a social network means that people with large networks of healthy adults have a better chance of receiving life-saving treatment than people without the same social privileges.[8]
See also
[edit]- Blood bank#History (history of blood donation)
- Blood donation restrictions on men who have sex with men
- Blood substitute
- James Harrison (blood donor)
- List of blood donation agencies
- World Blood Donor Day
- Xenotransfusion
References
[edit]- ^ "Questions About Donating Blood". Blood Services. American Red Cross. Archived from the original on 17 September 2024. Retrieved 18 September 2024.
- ^ M. E. Brecher, Editor (2005), AABB Technical Manual, 15th ed., Bethesda, MD: AABB, ISBN 1-56395-196-7, pp. 98–103
- ^ "Directed Donation". Mayo Clinic. Archived from the original on 2008-05-24. Retrieved 2008-06-25.
- ^ Wales PW, Lau W, Kim PC (May 2001). "Directed blood donation in pediatric general surgery: Is it worth it?". J. Pediatr. Surg. 36 (5): 722–725. doi:10.1053/jpsu.2001.22945. PMID 11329574.
- ^ a b T. Brown "Strengthening Blood Systems In Africa: Progress Under PEPFAR and Remaining Challenges" AABB News. April 1998: p. 30
- ^ "Autologous (self-donated) Blood as an Alternative to Allogeneic (donor-donated) Blood Transfusion". AABB. Archived from the original on 2008-06-12. Retrieved 2008-06-25.
- ^ "Recovered Plasma". AABB. Archived from the original on 2008-06-12. Retrieved 2008-06-25.
- ^ a b c d e f g h i j k l m n o Weaver, Meaghann S.; Yee, Marianne E. M.; Lawrence, Courtney E.; Fasano, Ross M. (2023-04-01). "Requests for Directed Blood Donations". Pediatrics. 151 (4): e2022058183. doi:10.1542/peds.2022-058183. ISSN 0031-4005. PMC 10998552. PMID 36897227.
- ^ Gribble DM, Chaffin DJ, Bryant BJ. Cost-effectiveness of FDA variance for blood collection from individuals with hereditary hemochromatosis at a 398-bed hospital-based donor center. Immunohematology. 2009;25(4):170-3. PMID: 20406025.
- ^ "Giving Blood -> What to Expect". Australian Red Cross Blood Service. Archived from the original on 2007-08-29. Retrieved 2007-10-06.
- ^ "The Donation Experience". Canadian Blood Services. Archived from the original on 2007-02-03. Retrieved 2006-12-17.
- ^ "Tips for a Good Donation Experience". American Red Cross. Archived from the original on 2006-12-14. Retrieved 2006-12-17.
- ^ "WHO Blood Safety and Donation". World Health Organization. Archived from the original on 2008-06-29. Retrieved 2008-06-01.
- ^ a b c "World Blood Donor Day 2006". World Health Organization. Archived from the original on 2008-06-17. Retrieved 2008-06-26.
- ^ "Sponsoring a Blood Drive". American Red Cross. Archived from the original on 2008-05-26. Retrieved 2008-06-25.
- ^ "Parental consent form" (PDF). Australian Red Cross Blood Service. Archived (PDF) from the original on 2008-06-25. Retrieved 2008-06-01.
- ^ "FDA regulations on donor deferral". US Food and Drug Administration. Archived from the original on 2008-05-14. Retrieved 2008-06-01.
- ^ "Variances for Blood Collection from Individuals with Hereditary Hemochromatosis". US Food and Drug Administration. Archived from the original on 2007-07-08. Retrieved 2007-07-18.
- ^ "Hereditary Hemochromatosis: Perspectives of Public Health, Medical Genetics, and Primary Care". CDC Office of Public Health Genomics. Archived from the original on 2008-03-08. Retrieved 2008-06-03.
- ^ Severo, Richard (1990-01-13). "Donors' Races to Be Sought To Identify Rare Blood Types". The New York Times. Archived from the original on 2009-03-03. Retrieved 2008-06-01.
- ^ "Red Gold, Innovators and Pioneers". Public Broadcasting Service (United States). Archived from the original on 2008-05-17. Retrieved 2008-06-01.
- ^ "How Science Students Helped End Segregated Blood Banks". National Center For Science Education (United States). 2015-08-05. Archived from the original on 2017-11-07. Retrieved 2017-11-05.
- ^ "Drug Agency Reaffirms Ban on Gay Men Giving Blood". The New York Times. 2007-05-24. Archived from the original on 2013-05-24. Retrieved 2009-03-26.
- ^ "HIV charities welcome the lifting of lifetime ban on gay men donating blood". National Aids Trust. 2011-09-08. Archived from the original on 2014-02-02. Retrieved 2014-01-31.
- ^ "Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products – Questions and Answers". US Food and Drug Administration. Archived from the original on 2016-03-30. Retrieved 2016-03-30.
- ^ "The rules on blood donation in England change on 28th November". NHS Blood and Transplant. 2017-11-28. Archived from the original on 2019-10-15. Retrieved 2019-10-21.
- ^ a b c "FDA policy allowing more gay and bisexual men to donate blood goes into effect". NBC News. 2023-08-07. Retrieved 2023-08-15.
- ^ Heim MU, Mempel W (1991). "[The need for thorough infection screening in donors of autologous blood]". Beitr Infusionsther (in German). 28: 313–316. PMID 1725645.
- ^ "Avodart consumer information". US Food and Drug Administration. Archived from the original on May 9, 2009. Retrieved 2008-06-01.
- ^ "Donor Qualification criteria". Héma-Québec, Canada. Archived from the original on 2006-11-08. Retrieved 2006-12-17.
- ^ "Permanent exclusion criteria / Dyskwalifikacja stała" (in Polish). RCKiK Warszawa. Archived from the original on August 30, 2009. Retrieved 2010-03-03.
- ^ "Guidelines for UK Blood Services". UK Blood and Tissue Services. Archived from the original on 2008-05-15. Retrieved 2008-06-01.
- ^ Hoad VC, Seed CR, Kiely P, Styles CE, McManus H, Law M, Kaldor J, Gosbell IB. Removal of UK-donor deferral for variant Creutzfeldt-Jakob disease: A large donation gain in Australia. Vox Sang. 2023 Oct;118(10):891-894. doi: 10.1111/vox.13510. Epub 2023 Aug 11. PMID: 37563946.
- ^ Gómez-Simón A; Navarro-Núñez L; Pérez-Ceballos E; et al. (Jun 2007). "Evaluation of four rapid methods for hemoglobin screening of whole blood donors in mobile collection settings". Transfus. Apher. Sci. 36 (3): 235–242. doi:10.1016/j.transci.2007.01.010. PMID 17556020.
- ^ "Iron Information for All Blood Donors". www.redcrossblood.org. Archived from the original on 2020-10-16. Retrieved 2020-10-20.
- ^ Goldman M, Fournier E, Cameron-Choi K, Steed T (May 2007). "Effect of changing the age criteria for blood donors". Vox Sang. 92 (4): 368–372. doi:10.1111/j.1423-0410.2007.00897.x. PMID 17456161. S2CID 36493554.
- ^ "Eligibility Requirements". www.redcrossblood.org. Archived from the original on 2020-10-16. Retrieved 2020-10-20.
- ^ "Donating – Frequently Asked Questions". Blood Bank of Alaska. Archived from the original on 2008-06-06. Retrieved 2008-06-01.
- ^ Dzik WH, Fleisher AG, Ciavarella D, Karlson KJ, Reed GE, Berger RL. Safety and efficacy of autologous blood donation before elective aortic valve operation. Ann Thorac Surg. 1992 Dec;54(6):1177-80; discussion 1180-1. doi: 10.1016/0003-4975(92)90090-q. PMID: 1449306.
- ^ "Blood Type Test". WebMD.com. Archived from the original on 2008-05-29. Retrieved 2008-06-01.
- ^ "Plasma fact sheet" (PDF). American Red Cross. Archived from the original (PDF) on 2009-03-26.
- ^ "Right Type, Right Time". Archived from the original on 2018-07-22. Retrieved 2018-07-22.
- ^ Bhattacharya P; Chandra PK; Datta S; et al. (Jul 2007). "Significant increase in HBV, HCV, HIV, and syphilis infections among blood donors in West Bengal, Eastern India 2004–2005: exploratory screening reveals high frequency of occult HBV infection". World J. Gastroenterol. 13 (27): 3730–3733. doi:10.3748/wjg.v13.i27.3730. PMC 4250646. PMID 17659734.
- ^ "Testing of Donor Blood for infectious disease". AABB. Archived from the original on 2008-05-09. Retrieved 2008-06-25.
- ^ R. Miller; P.E. Hewitt; R. Warwick; M.C. Moore; B. Vincent (1998). "Review of counselling in a transfusion service: the London (UK) experience". Vox Sang. 74 (3): 133–139. doi:10.1046/j.1423-0410.1998.7430133.x. PMID 9595639. S2CID 24514380.
- ^ "Blood safety and availability". www.who.int. Archived from the original on 2020-04-24. Retrieved 2022-10-28.
- ^ "Advisory Committee on MSBTO, 28 June 2005" (PDF). Archived from the original (PDF) on 28 May 2008. Retrieved 2008-06-01.
- ^ "Precautionary West Nile virus blood sample testing". Héma-Québec, Canada. Archived from the original on 2007-09-13. Retrieved 2006-12-17.
- ^ "FDA approves first tests to screen for tickborne parasite in whole blood and plasma to protect the U.S. blood supply" (Press release). FDA. 6 March 2018. Archived from the original on 7 March 2018. Retrieved 6 March 2018.
While babesiosis is both preventable and treatable, until today, there was no way to screen for infections amongst blood donors
- ^ a b c "Circular of Information for use of Blood and Blood Products" (PDF). AABB, ARC, America's Blood Centers. Archived from the original (PDF) on 2009-10-07.
- ^ "Red blood cell transfusions in newborn infants: Revised guidelines". Canadian Paediatric Society (CPS). Archived from the original on 2007-02-03. Retrieved 2007-02-02.
- ^ a b Kim, Daniel H.; Berkman, Emily; Clark, Jonna D.; Saifee, Nabiha H.; Diekema, Douglas S.; Lewis-Newby, Mithya (2023). "Parental Refusals of Blood Transfusions from COVID-19 Vaccinated Donors for Children Needing Cardiac Surgery". Narrative Inquiry in Bioethics. 13 (3): 215–226. doi:10.1353/nib.2023.a924193. ISSN 2157-1740. PMID 38661995.
- ^ Coulthard SL, Kaplan LJ, Cannon JW. What's new in whole blood resuscitation? In the trauma bay and beyond. Curr Opin Crit Care. 2024 Jun 1;30(3):209-216. doi: 10.1097/MCC.0000000000001140. Epub 2024 Feb 6. PMID: 38441127.
- ^ a b "Why Should I Donate Blood". WHO. 2022-06-06. Archived from the original on 2023-02-18. Retrieved 2023-02-18.
- ^ "How Much Is One Unit of Blood?". reference.com. Ask Media Group LLC. 2020-04-11. Retrieved 2023-02-18.
- ^ a b "Blood donation: What to expect". Mayo Clinic. Archived from the original on 2008-12-04. Retrieved 2008-12-03.
- ^ Eberts, Dan (2021-08-17). "How Much Blood Is Taken When You Donate?". OneBlood. Archived from the original on 2023-02-18. Retrieved 2023-02-18.
- ^ Commission Directive 2004/33/EC (Report). Official Journal of the European Union. 2004-03-22. p. 14. Retrieved 2023-02-18.
- ^ "Donation Process". National AIDS Control Organisation. National Blood Transfusion Council. Archived from the original on 2023-02-18. Retrieved 2023-02-18.
- ^ "Blood facts and figures". Government of Singapore. Health Sciences Authority. 31 October 2018. Archived from the original on 2023-02-18. Retrieved 2023-02-18.
- ^ 献血者血液検査結果の集計 / Hematologic and Biochemical Values in Blood Donors (PDF) (Report). Japanese Red Cross Society. 2017. p. 5. Retrieved 2023-02-18.
- ^ Jingxing Wang; Nan Guo (corresponding); et al. (December 2010). "Who donates blood at five ethnically and geographically diverse blood centers in China in 2008". Transfusion. 50 (12): 2686–2694. doi:10.1111/j.1537-2995.2010.02722.x. PMID 20553435. S2CID 205723043.
- ^ Waxman DA (2002). "Volunteer donor apheresis". Ther Apher. 6 (1): 77–81. doi:10.1046/j.1526-0968.2002.00395.x. PMID 11886580.
- ^ Sagi E, Eyal F, Armon Y, Arad I, Robinson M (Nov 1981). "Exchange transfusion in newborns via a peripheral artery and vein". Eur. J. Pediatr. 137 (3): 283–284. doi:10.1007/BF00443258. PMID 7318840. S2CID 23219586.
- ^ "Blood on the Hoof". Public Broadcasting Service. Archived from the original on 2008-06-04. Retrieved 2008-06-25.
- ^ "ISBT Quarterly Newsletter, June 2006, "A History of Fresh Blood", p. 15" (PDF). International Society of Blood Transfusion (ISBT/SITS). Archived from the original (PDF) on 2008-08-03. Retrieved 2008-07-31.
- ^ a b Lee CK, Ho PL, Chan NK, Mak A, Hong J, Lin CK (Oct 2002). "Impact of donor arm skin disinfection on the bacterial contamination rate of platelet concentrates". Vox Sang. 83 (3): 204–208. doi:10.1046/j.1423-0410.2002.00219.x. PMID 12366760. S2CID 19946631.
- ^ M. L. Turgeon (2004). Clinical Hematology: Theory and Procedures (fourth ed.). Lippincott Williams & Wilkins. p. 30. ISBN 978-0-7817-5007-3. Retrieved 2008-06-21.
- ^ One major manufacturer of collection sets uses a 16 gauge (1.651 mm) size "Blood banking laboratory supplies" (PDF). Genesis BPS. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-01.
- ^ "What is Hemolysis?" (PDF). Becton-Dickinson. Archived (PDF) from the original on 2008-06-25. Retrieved 2008-06-01.
- ^ Akerblom O, Kreuger A (1975). "Studies on citrate-phosphate-dextrose (CPD) blood supplemented with adenine". Vox Sang. 29 (2): 90–100. doi:10.1111/j.1423-0410.1975.tb00484.x. PMID 238338. S2CID 9785373.
- ^ Sugita, Yoshiki; Simon, Ernest R. (1965). "The Mechanism of Action of Adenine in Red Cell Preservation*". Journal of Clinical Investigation. 44 (4): 629–642. doi:10.1172/JCI105176. ISSN 0021-9738. PMC 292538. PMID 14278179.
- ^ Simon, Ernest R.; Chapman, Robert G.; Finch, Clement A. (1962). "Adenine in red cell preservation". Journal of Clinical Investigation. 41 (2): 351–359. doi:10.1172/JCI104489. ISSN 0021-9738. PMC 289233. PMID 14039291.
- ^ "Plasma Equipment and Packaging, and Transfusion Equipment". Office of Medical History (OTSG). Archived from the original on 2017-06-09. Retrieved 2008-06-19.
- ^ "Medicines derived from human plasma". Sanquin Blood Supply Foundation. Archived from the original on 2009-01-01. Retrieved 2008-06-01.
- ^ Component Donation Archived 2009-10-11 at the Wayback Machine UK National Blood Service. Retrieved 2009-10-26
- ^ "Indications for Platelet Transfusion Therapy". Southeastern Community Blood Center. Archived from the original on 2008-05-12. Retrieved 2008-06-10.
- ^ "Platelet Donation". The American National Red Cross. Archived from the original on 2019-03-29. Retrieved 2019-05-16.
- ^ "Double Up to Save Lives". United Blood Services. Archived from the original on 2006-12-30. Retrieved 2007-02-23.
- ^ "Double the Difference". American Red Cross (Greater Chesapeake and Potomac). Archived from the original on 2007-05-13. Retrieved 2007-02-23.
- ^ Eder AF, Hillyer CD, Dy BA, Notari EP, Benjamin RJ (May 2008). "Adverse reactions to allogeneic whole blood donation by 16- and 17-year-olds". JAMA. 299 (19): 2279–2286. doi:10.1001/jama.299.19.2279. PMID 18492969.
- ^ "Report on the promotion by Member States of voluntary unpaid blood donation" (PDF). Commission of the European Communities. Archived from the original (PDF) on 2008-08-03. Retrieved 2008-06-26.
- ^ "Donating Apheresis and Plasma". Community Blood Center. Archived from the original on 2008-07-04. Retrieved 2008-06-11.
- ^ Pottgiesser T, Specker W, Umhau M, Dickhuth HH, Roecker K, Schumacher YO (2008). "Recovery of hemoglobin mass after blood donation". Transfusion. 48 (7): 1390–1397. doi:10.1111/j.1537-2995.2008.01719.x. PMID 18466177. S2CID 21814989.
- ^ "Blood Products Advisory Committee, 12 December 2003". Food and Drug Administration. Archived from the original on 8 November 2008. Retrieved 2008-06-01.
- ^ Smith GA, Fisher SA, Doree C, Di Angelantonio E, Roberts DJ (2014). "Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors". Cochrane Database Syst Rev. 2014 (7): CD009532. doi:10.1002/14651858.CD009532.pub2. PMC 11019466. PMID 24990381. S2CID 205200473.
- ^ A.F. Eder; C.D. Hillyer; B.A. Dy; E.P. Notari; R.J. Benjamin (May 2008). "Adverse reactions to allogeneic whole blood donation by 16- and 17-year-olds". JAMA. 299 (19): 2279–2286. doi:10.1001/jama.299.19.2279. PMID 18492969.
- ^ Yuan S, Gornbein J, Smeltzer B, Ziman AF, Lu Q, Goldfinger D (Jun 2008). "Risk factors for acute, moderate to severe donor reactions associated with multicomponent apheresis collections". Transfusion. 48 (6): 1213–1219. doi:10.1111/j.1537-2995.2008.01674.x. PMID 18346014. S2CID 403463.
- ^ "Adverse Effect of Blood Donation, Siriraj Experience" (PDF). American Red Cross. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-01.
- ^ B. Newman; S. Graves (2001). "A study of 178 consecutive vasovagal syncopal reactions from the perspective of safety". Transfusion. 41 (12): 1475–1479. doi:10.1046/j.1537-2995.2001.41121475.x. PMID 11778059. S2CID 23789257.
- ^ "Fatalities Reported to FDA". US Food and Drug Administration. Archived from the original on 2011-01-02. Retrieved 2010-12-20.
- ^ Wiltbank TB, Giordano GF, Kamel H, Tomasulo P, Custer B (May 2008). "Faint and prefaint reactions in whole-blood donors: an analysis of predonation measurements and their predictive value". Transfusion. 48 (9): 1799–1808. doi:10.1111/j.1537-2995.2008.01745.x. PMID 18482188. S2CID 157599.
- ^ Berg, P; Heiden, M; Müller, S; Meyer, B; Witzenhausen, C; Ruppert-Seipp, G; Kehr, S; Funk, MB (2024-06-18). "A national surveillance system for continuous monitoring of blood transfusion safety: German haemovigilance data". Vox Sanguinis. 119 (9): 953–962. doi:10.1111/vox.13694. PMID 38889998.
- ^ Orru', S; Poetzsch, K; Hoffelner, M; Heiden, M; Funk, MB; Keller-Stanislawski, B; Oberle, D (2021). "Blood Donation-Related Adverse Reactions: Results of an Online Survey among Donors in Germany (2018)". Transfusion Medicine and Hemotherapy. 48 (5): 272–283. doi:10.1159/000516049. PMC 8578804. PMID 34803571.
- ^ Ranasinghe E, Harrison JF (Jun 2000). "Bruising following blood donation, its management and the response and subsequent return rates of affected donors". Transfus Med. 10 (2): 113–116. doi:10.1046/j.1365-3148.2000.00240.x. PMID 10849380. S2CID 46557010.
- ^ Working Group on Complications Related to Blood Donation JF (2008). "Standard for Surveillance of Complications Related to Blood D Donation" (PDF). European Haemovigilance Network: 11. Archived from the original (PDF) on 2010-02-15.
- ^ Bolan CD, Greer SE, Cecco SA, Oblitas JM, Rehak NN, Leitman SF (Sep 2001). "Comprehensive analysis of citrate effects during plateletpheresis in normal donors". Transfusion. 41 (9): 1165–1171. doi:10.1046/j.1537-2995.2001.41091165.x. PMID 11552076. S2CID 22220301. Archived from the original on 2020-07-27. Retrieved 2019-07-05.
- ^ Sorensen, BS; Johnsen, SP; Jorgensen, J. (Feb 2008). "Complications related to blood donation: A population-based study". Vox Sanguinis. 94 (2): 132–137. doi:10.1111/j.1423-0410.2007.01000.x. PMID 18028259. S2CID 35763171.
- ^ "Jerome H. Holland Laboratory for the Biomedical Sciences Volunteer Research Blood Program (RBP)". American Red Cross. Archived from the original on 2008-03-15. Retrieved 2008-06-01.
- ^ Wiltbank TB, Giordano GF; Giordano, Gerald F. (Jun 2007). "The safety profile of automated collections: an analysis of more than 1 million collections". Transfusion. 47 (6): 1002–1005. doi:10.1111/j.1537-2995.2007.01224.x. PMID 17524089. S2CID 19739978.
- ^ Global AIDS Crisis: A Reference Handbook, Richard G. Marlink, Alison G. Kotin, p. 16 [1], ABC-CLIO
- ^ "Blood Donor Information Leaflet". Irish Blood Transfusion Service. Archived from the original on 2007-11-19. Retrieved 2008-06-01.
- ^ "Keeping China's blood supply free of HIV". US Embassy, Beijing. Archived from the original on 2008-09-07. Retrieved 2008-06-01.
- ^ Cohen J (Jun 2004). "HIV/AIDS in China. An unsafe practice turned blood donors into victims". Science. 304 (5676): 1438–1439. doi:10.1126/science.304.5676.1438. PMID 15178781. S2CID 45756186.
- ^ Busby, Mattha (26 September 2019). "Contaminated blood whistleblower dies in US". The Guardian. Archived from the original on 26 September 2019. Retrieved 26 September 2019.
- ^ Beard, M.; Garwood, M.; Cookson, P.; Bashir, S.; Hancock, V.; Pergande, C.; Smith, K.; Turner, C.; Wiltshire, M.; Thomas, S.; Cardigan, R. (2006). "In Vitro Evaluation of Buffy Coat Derived Platelet Concentrates in SSP+ Platelet Storage Medium". Transfusion Medicine. 16: 26. doi:10.1111/j.1365-3148.2006.00694_4.x. S2CID 72452243.
- ^ "Transfusion Handbook, summary information for Platelets". National Blood Transfusion Committee. Archived from the original on 2008-08-04. Retrieved 2008-06-02.
- ^ Lockwood WB, Hudgens RW, Szymanski IO, Teno RA, Gray AD (Nov 2003). "Effects of rejuvenation and frozen storage on 42-day-old AS-3 RBCs". Transfusion. 43 (11): 1527–1532. doi:10.1046/j.1537-2995.2003.00551.x. PMID 14617310. S2CID 22694513.
- ^ "Transfusion handbook, Summary information for Red Blood Cells". National Blood Transfusion Committee. Archived from the original on 2008-08-04. Retrieved 2008-06-02.
- ^ "Transfusion of Fresh Frozen Plasma, products, indications" (PDF). Agence française de sécurité sanitaire des produits de santé. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-02.
- ^ "American Red Cross Issues Emergency Need for Blood Donors". www.redcross.org. Archived from the original on 2020-08-15. Retrieved 2020-12-12.
- ^ Glynn, Simone A.; Williams, Alan E.; Nass, Catharie C.; Bethel, James; Kessler, Debra; Scott, Edward P.; Fridey, Joy; Kleinman, Steven H.; Schreiber, George B.; Retrovirus Epidemiology Donor Study (January 2003). "Attitudes toward blood donation incentives in the United States: implications for donor recruitment". Transfusion. 43 (1): 7–16. doi:10.1046/j.1537-2995.2003.00252.x. PMID 12519425. S2CID 25995392.
- ^ a b McCarthy, Leo J. (May 2007). "How do I manage a blood shortage in a transfusion service?". Transfusion. 47 (5): 760–762. doi:10.1111/j.1537-2995.2007.01187.x. ISSN 0041-1132. PMID 17465938. S2CID 32044726.
- ^ Hensley, Scott (October 25, 1999). "Blood Shortages Become More Common". Modern Healthcare. 29: 34. Archived from the original on April 15, 2021. Retrieved April 15, 2021.
- ^ a b c Sass, Reuben G. (June 2013). "Toward a More Stable Blood Supply: Charitable Incentives, Donation Rates, and the Experience of September 11". The American Journal of Bioethics. 13 (6): 38–45. doi:10.1080/15265161.2013.781703. ISSN 1526-5161. PMID 23641850. S2CID 29044770.
- ^ Schmidt, Paul J. (2002-02-21). "Blood and Disaster – Supply and Demand". New England Journal of Medicine. 346 (8): 617–620. doi:10.1056/nejm200202213460813. ISSN 0028-4793. PMID 11856803.
- ^ a b c d Glynn, Simone A.; Busch, Michael P.; Schreiber, George B.; Murphy, Edward L.; Wright, David J.; Tu, Yongling; Kleinman, Steven H.; for the NHLBI REDS Study Group (2003-05-07). "Effect of a National Disaster on Blood Supply and Safety: The September 11 Experience". JAMA. 289 (17): 2246–2253. doi:10.1001/jama.289.17.2246. ISSN 0098-7484. PMID 12734136. S2CID 22399331.
- ^ "Maintaining an Adequate Blood Supply Is Key to Emergency Preparedness" (PDF). Government Accountability Office. Archived from the original (PDF) on 2013-12-24. Retrieved 2008-06-01.
- ^ "Current status of America's Blood Centers blood supply". America's Blood Centers. Archived from the original on 2009-02-01.
- ^ "World Blood Donor Day". World Health Organization. Archived from the original on 2008-09-15. Retrieved 2008-06-01.
- ^ "Blood safety and availability". World Health Organization. Archived from the original on 2018-04-18. Retrieved 2019-01-11.
- ^ Riley W, Schwei M, McCullough J (July 2007). "The United States' potential blood donor pool: estimating the prevalence of donor-exclusion factors on the pool of potential donors" (PDF). Transfusion. 47 (7): 1180–1188. doi:10.1111/j.1537-2995.2007.01252.x. PMID 17581152. S2CID 20867091. Archived from the original (PDF) on 2012-09-27. Retrieved 2013-01-04.
- ^ a b "Facts About Blood". America's Blood Centers. Archived from the original on 2013-11-08. Retrieved 2013-01-04.
- ^ "Give Blood". NHS Blood & Transfustion. Archived from the original on 2011-12-30. Retrieved 2012-01-04.
Blood is something we all expect to be there for us when we need it, yet only 4% of us give blood.
- ^ "Canadian Blood Services". Archived from the original on 2013-05-23.
- ^ Edwards, Patrick W.; Zeichner, Amos (January 1985). "Blood donor development: Effects of personality, motivational and situational variables". Personality and Individual Differences. 6 (6): 743–751. doi:10.1016/0191-8869(85)90085-6. ISSN 0191-8869.
- ^ a b Muthivhi, Tshilidzi; Olmsted, M.; Park, H.; Sha, Mandy (August 2015). "Motivators and deterrents to blood donation among Black South Africans: a qualitative analysis of focus group data". Transfusion Medicine. 25 (4): 249–258. doi:10.1111/tme.12218. ISSN 0958-7578. PMC 4583344. PMID 26104809.
- ^ Drake AW. "Public Attitudes and Decision Processes With Regard to Blood Donation: Final Report and Executive Summary". Cambridge, Mass: MIT; 1978:1–189.
- ^ Fields AC, Grindon AJ (2020). "Hemochromatosis, iron, and blood donation: a short review". Immunohematology. 15 (3): 108–112. doi:10.21307/immunohematology-2019-628. PMID 15373512. S2CID 23137986.
- ^ Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT (Mar 1997). "Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland". BMJ. 314 (7083): 793–794. doi:10.1136/bmj.314.7083.793. PMC 2126176. PMID 9080998.
- ^ Atsma, F.; de Vegt, F. (Sep 2011). "The healthy donor effect: a matter of selection bias and confounding". Transfusion. 51 (9): 1883–1885. doi:10.1111/j.1537-2995.2011.03270.x. PMID 21790637. S2CID 30195641.
- ^ Manco, Melania; Fernandez-Real, Josè (1 January 2012). "Back to past leeches: repeated phlebotomies and cardiovascular risk". BMC Medicine. 10 (1): 53. doi:10.1186/1741-7015-10-53. PMC 3409018. PMID 22647488.

- ^ Matei, Adrienne (12 May 2022). "Here's another reason to donate blood: it reduces 'forever chemicals' in your body". The Guardian. Guardian News & Media Limited. Archived from the original on 24 May 2022. Retrieved 24 May 2022.
- ^ "Blood Plasma Safety" (PDF). GAO. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-01.
- ^ "Donate Blood For Money". Archived from the original on 2014-12-18.
- ^ L. Fusco "From Latin America to Asia, Rising Above Difficulties, Achieving New Heights" AABB News. April 1998: p. 30
- ^ "Guidelines for Implementation of Employee Blood Donation Leave" (PDF). New York State Department of Labor. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-06-01.
- ^ "Legge 21 ottobre 2005, n. 219 (Law 21 October 2005, n. 219)". Italian Parliament. Archived from the original on 27 April 2009. Retrieved 2009-09-04.
- ^ "Dwa dni wolnego od pracy dla honorowego dawcy na stałe w przepisach". 11 August 2023.
- ^ "Incentives program for blood donors and organizers". American Red Cross Connecticut Blood Services Region. Archived from the original on 2008-06-02. Retrieved 2008-06-01.
- ^ "Red Cross Honour Roll". Archived from the original on 2012-09-28.
- ^ "Are you one of the 1.2m?". 5 February 2004. Archived from the original on 2023-04-11. Retrieved 2023-04-11.
- ^ "Keistimewaan Penderma Darah" [Blood Donor Privileges]. Archived from the original on 2021-04-15. Retrieved 2021-04-15.
- ^ "Ceny I Zasady Kupna Biletów".
- ^ "Donate blood, get antibody test". Queens Chronicle. 25 February 2021. Archived from the original on 26 February 2021. Retrieved 26 March 2021.
- ^ Dunbar, Erin (1 March 2021). "Blood drive in Huntsville offering free COVID-19 antibody test". WHNT 19. Archived from the original on 1 March 2021. Retrieved 26 March 2021.
- ^ "Red Cross calls on donors to refuel the blood supply". The Star. 25 March 2021. Archived from the original on 26 March 2021. Retrieved 26 March 2021.
- ^ Steele WR; Schreiber GB; Guiltinan A; et al. (Jan 2008). "The role of altruistic behavior, empathetic concern, and social responsibility motivation in blood donation behavior". Transfusion. 48 (1): 43–54. doi:10.1111/j.1537-2995.2007.01481.x. PMID 17894795. S2CID 19252616.
- ^ "Richard Titmuss". National University of Taipei social work department. Archived from the original on 2011-01-15. Retrieved 2010-11-07.
- ^ Catherine Waldby, The University of Sydney. "The logistics of altruism". Archived from the original on 2011-01-21. Retrieved 2010-11-07.
- ^ Philippe Steiner (2003). "Gifts of Blood and Organs: the Market and "Fictitious" Commodities". Revue Française de Sociologie (5). Revue française de sociologie 5/2003 (Volume 44), pp. 147–162: 147–162. doi:10.3917/rfs.445.0147. Archived from the original on 2011-12-04. Retrieved 2010-11-07.
Further reading
[edit]- Deferred Donors: Anemia & Blood Donation
- British guidelines for transfusion medicine
- Harrison E (Nov 2007). "Blood cells for sale". Sci. Am. 297 (5): 108–109. Bibcode:2007SciAm.297e.108H. doi:10.1038/scientificamerican1107-108. PMID 17990831.
(subtitle) There's more to blood banking than just bagging blood
