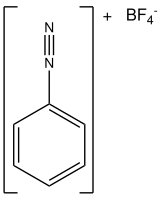
Benzenediazonium tetrafluoroborate
| |

| |
| Names | |
|---|---|
| IUPAC name
Benzenediazonium tetrafluoroborate
| |
| Other names
Phenyldiazonium tetrafluoroborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6H5BF4N2 | |
| Molar mass | 191.92 g·mol−1 |
| Appearance | colorless crystals |
| Density | 1.565 g/cm3 |
| Melting point | decomposes |
| Boiling point | decomposes |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzenediazonium tetrafluoroborate is an organic compound with the formula [C6H5N2]BF4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the aryldiazonium compounds,[1] which are widely used in organic chemistry.
Synthesis
[edit]Diazotization of aniline in the presence of hydrochloric acid:
- C6H5NH2 + HNO2 + HCl → [C6H5N2]Cl + 2 H2O
The tetrafluoroborate can be obtained from crude benzenediazonium chloride by salt metathesis using tetrafluoroboric acid.
- [C6H5N2]Cl + HBF4 → [C6H5N2]BF4 + HCl
The tetrafluoroborate is more stable than the chloride.[2]
Properties
[edit]The diazo group (N2) can be replaced by many other groups, usually anions, giving a variety of substituted phenyl derivatives:
- C6H5N2+ + Nu− → C6H5Nu + N2
These transformations are associated with many named reactions including the Schiemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide range of groups that can be used to replace N2 including halide, SH−, CO2H−, OH−. Of considerable practical value in the dye industry are the diazo coupling reactions.
The reaction of phenyldiazonium salts with aniline gives 1,3-diphenyltriazene.[3]
The structure of the salt has been verified by X-ray crystallography. The N-N bond distance is 1.083(3) Å.[4]
Safety
[edit]Whereas the chloride salt is explosive,[5] the tetrafluoroborate is readily isolated.
References
[edit]- ^ March, J. (1992). Advanced Organic Chemistry (4th ed.). New York: J. Wiley and Sons. ISBN 0-471-60180-2.
- ^ Flood, D. T. (1933). "Fluorobenzene". Org. Synth. 13: 46. doi:10.15227/orgsyn.013.0046.
- ^ Hartman, W. W.; Dickey, J. B. (1934). "Diazoaminobenzene". Organic Syntheses. 14: 24. doi:10.15227/orgsyn.014.0024.
- ^ Cygler, Miroslaw; Przybylska, Maria; Elofson, Richard Macleod (1982). "The Crystal Structure of Benzenediazonium Tetrafluoroborate, C6H5N2+•BF4−1". Canadian Journal of Chemistry. 60 (22): 2852–2855. doi:10.1139/v82-407.
- ^ Nesmajanow, A. N. (1932). "β-Naphthylmercuric chloride". Organic Syntheses. 12: 54; Collected Volumes, vol. 2, p. 432.
