Triammonium citrate
Appearance
(Redirected from Ammonium citrate)
This article needs additional citations for verification. (November 2023) |
| |

| |
| Names | |
|---|---|
| IUPAC name
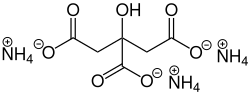
Ammonium 2-hydroxypropane-1,2,3-tricarboxylate
| |
| Other names
Ammonium citrate tribasic; Ammonium citrate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.020.359 |
| EC Number |
|
| E number | E380 (antioxidants, ...) |
PubChem CID
|
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H17N3O7 | |
| Molar mass | 243.216 g·mol−1 |
| Density | 1.48 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triammonium citrate is a chemical compound whose molecular formula is C6H17N3O7.[1]
Synopsis
[edit]It was patented some date prior to 1986.[2]
This substance causes serious eye irritation, causes skin irritation and may cause respiratory irritation.[1]
It is known in the European E number food additive series as E380. It is known in the United States as "an indirect food additive for use only as a component of adhesives", and as a "substance added directly to human food affirmed as generally recognized as safe (GRAS)."[2]
References
[edit]- ^ a b "Substance information - Triammonium citrate". European Chemicals Agency.
- ^ a b "Compound Summary for CID 18954 - Ammonium Citrate, Tribasic". PubChem.
