Alizarine Yellow R
Appearance
(Redirected from Alizarin Yellow R)
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
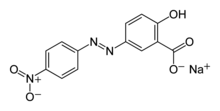
Sodium 2-hydroxy-5-[(E)-(4-nitrophenyl)diazenyl]benzoate
| |
| Other names
5-[(p-Nitrophenyl)azo]salicylic acid sodium salt
Chrome orange Mordant orange 1 C.I. 14030 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.017.109 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H8N3NaO5 (Na salt) C13H9N3O5 (acid) | |
| Molar mass | 309.21 g mol−1 (Na salt) 287.23 g mol−1 (acid) |
| HazardsSigma-Aldrich Co., ALIZARINE YELLOW R. Retrieved on 09 April 2023. | |
| GHS labelling: | |

| |
| Warning | |
| H302, H319 | |
| P264, P270, P280, P301+P312, P305+P351+P338, P330, P337+P313, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Alizarine Yellow R (pH indicator) | ||
| below pH 10.1 | above pH 12.0 | |
| 10.1 | ⇌ | 12.0 |
Alizarine Yellow R is a yellow colored azo dye made by the diazo coupling reaction. It is usually commercially available as a sodium salt. In its pure form, it is a rust-colored solid.[2] It is mainly used as a pH indicator.
Preparation
[edit]Alizarine Yellow R is produced by azo coupling of salicylic acid and diazonium derivative of 4-Nitroaniline
References
[edit]- ^ Lide, David R. (25 June 2007). CRC Handbook of Chemistry and Physics, 88th Edition. CRC Press. pp. 3–10. ISBN 9780849304880. OCLC 1024315229.
- ^ "Safety Datasheet (MSDS) for alizarin yellow R". Department of Chemistry, University of Oxford. 2005. Archived from the original on 19 March 2011. Retrieved 11 October 2008.

