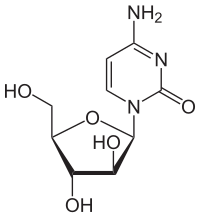
Cytarabine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cytosar-U, Depocyt, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682222 |
| Pregnancy category |
|
| Routes of administration | injectable (intravenous injection or infusion, intrathecal, or subcutaneously) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 20% by mouth |
| Protein binding | 13% |
| Metabolism | Liver |
| Elimination half-life | biphasic: 10 min, 1–3 hr |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.188 |
| Chemical and physical data | |
| Formula | C9H13N3O5 |
| Molar mass | 243.219 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cytarabine, also known as cytosine arabinoside (ara-C), is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin's lymphoma.[2] It is given by injection into a vein, under the skin, or into the cerebrospinal fluid.[2] There is a liposomal formulation for which there is tentative evidence of better outcomes in lymphoma involving the meninges.[2]
Common side effects include bone marrow suppression, vomiting, diarrhea, liver problems, rash, ulcer formation in the mouth, and bleeding.[2] Other serious side effects include loss of consciousness, lung disease, and allergic reactions.[2] Use during pregnancy may harm the baby.[2] Cytarabine is in the antimetabolite and nucleoside analog families of medication.[3] It works by blocking the function of DNA polymerase.[2]
Cytarabine was patented in 1960 and approved for medical use in 1969.[4] It is on the World Health Organization's List of Essential Medicines.[5]
Medical uses
[edit]Cytarabine is mainly used in the treatment of acute myeloid leukaemia, acute lymphocytic leukaemia (ALL) and in lymphomas,[6] where it is the backbone of induction chemotherapy.
Cytarabine also possesses antiviral activity, and it has been used for the treatment of generalised herpesvirus infection. However, cytarabine is not very selective in this setting and causes bone marrow suppression and other severe side effects. Therefore, ara-C is not a useful antiviral agent in humans because of its toxic profile.[7]
Cytarabine is also used in the study of the nervous system to control the proliferation of glial cells in cultures, the amount of glial cells having an important impact on neurons.[citation needed] Recently, cytarabine was reported to promote robust and persistent neuronal differentiation in NSC-34 motor neuron-like cell line. Cytarabine is permissive, dispensable, and mostly irreversible in priming NSC-34 cells for neurite initiation and regeneration after mechanical dislodgement.[8]
Side effects
[edit]One of the unique toxicities of cytarabine is cerebellar toxicity when given in high doses, which may lead to ataxia. Cytarabine may cause granulocytopenia and other impaired body defenses, which may lead to infection, and thrombocytopenia, which may lead to hemorrhage.[citation needed]
Toxicity: pancreatitis, leukopenia, thrombocytopenia, anemia, GI disturbances, stomatitis, conjunctivitis, pneumonitis, fever, and dermatitis, palmar-plantar erythrodysesthesia. Rarely, myelopathy has been reported after high dose or frequent intrathecal Ara-C administration.[9]
When used in protocols designated as high dose, cytarabine can cause cerebral and cerebellar dysfunction, ocular toxicity, pulmonary toxicity, severe GI ulceration and peripheral neuropathy (rare).[citation needed]
To prevent the side effects and improve the therapeutic efficiency, various derivatives of these drugs (including amino acid, peptide, fatty acid and phosphates) have been evaluated, as well as different delivery systems.[10]
Mechanism of action
[edit]Cytosine arabinoside combines a cytosine base with an arabinose sugar. It is an antimetabolic agent with the chemical name of 1β-arabinofuranosylcytosine. Certain sponges, where similar compounds were originally found, use arabinoside sugars for chemical defense.[11] Cytosine arabinoside is similar enough to human deoxycytosine to be incorporated into human DNA, but different enough that it kills the cell. Cytosine arabinoside interferes with the synthesis of DNA. Its mode of action is due to its rapid conversion into cytosine arabinoside triphosphate, which damages DNA when the cell cycle holds in the S phase (synthesis of DNA). Rapidly dividing cells, which require DNA replication for mitosis, are therefore most affected. Cytosine arabinoside also inhibits both DNA[12] and RNA polymerases and nucleotide reductase enzymes needed for DNA synthesis. Cytarabine is the first of a series of cancer drugs that altered the sugar component of nucleosides. Other cancer drugs modify the base.[13]
Cytarabine is often given by continuous intravenous infusion, which follows a biphasic elimination – initial fast clearance rate followed by a slower rate of the analog.[14] Cytarabine is transported into the cell primarily by hENT-1.[15] It is then monophosphorylated by deoxycytidine kinase and eventually cytarabine-5´-triphosphate, which is the active metabolite being incorporated into DNA during DNA synthesis.[citation needed]
Several mechanisms of resistance have been reported.[16] Cytarabine is rapidly deaminated by cytidine deaminase in the serum into the inactive uracil derivative. Cytarabine-5´-monophosphate is deaminated by deoxycytidylate deaminase, leading to the inactive uridine-5´-monophosphate analog.[17] Cytarabine-5´-triphosphate is a substrate for SAMHD1.[18] Furthermore, SAMHD1 has been shown to limit the efficacy of cytarabine efficacy in patients.[19]
When used as an antiviral, cytarabine-5´-triphosphate functions by inhibiting viral DNA synthesis.[20] Cytarabine is able to inhibit herpesvirus and vaccinia virus replication in cells during tissue culture. However, cytarabine treatment was only effective for herpesvirus infection in a murine model.[citation needed]
In mice, Ara-CTP (cytarabine-5'-triphosphate) blocks memory consolidation, but not short-term memory, of a context fear conditioning event.[21] The blockage of memory consolidation was proposed to be due to the inhibition by Ara-CTP of the DNA non-homologous end joining pathway.[21] Thus transient DNA breakage followed by non-homologous end joining appear to be necessary steps in the formation of a long-term memory of an event.[citation needed]
History
[edit]Isolation of arabinose-containing nucleotides from the Caribbean sponge Cryptotheca crypta (now Tectitethya crypta) together with the realization that these compounds could act as DNA synthesis chain terminators led to exploration of these novel nucleotides as potential anticancer therapeutics.[22] Cytarabine was first synthesized in 1959 by Richard Walwick, Walden Roberts, and Charles Dekker at the University of California, Berkeley.[23]
It was approved by the United States Food and Drug Administration in June 1969, and was initially marketed in the US by Upjohn under the brand name Cytosar-U.[citation needed]
Names
[edit]It is also known as ara-C (arabinofuranosyl cytidine).[24]
- Cytosar-U
- Tarabine PFS (Pfizer)
- Depocyt (longer-lasting liposomal formulation)
- AraC
References
[edit]- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f g "Cytarabine". The American Society of Health-System Pharmacists. Archived from the original on 11 June 2016. Retrieved 8 December 2016.
- ^ British national formulary: BNF 69 (69 ed.). British Medical Association. 2015. p. 589. ISBN 9780857111562.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 511. ISBN 9783527607495. Archived from the original on 2016-12-20.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Pigneux A, Perreau V, Jourdan E, Vey N, Dastugue N, Huguet F, et al. (October 2007). "Adding lomustine to idarubicin and cytarabine for induction chemotherapy in older patients with acute myeloid leukemia: the BGMT 95 trial results". Haematologica. 92 (10): 1327–1334. doi:10.3324/haematol.11068. PMID 18024370.
- ^ Lauter CB, Bailey EJ, Lerner AM (November 1974). "Assessment of cytosine arabinoside as an antiviral agent in humans". Antimicrobial Agents and Chemotherapy. 6 (5): 598–602. doi:10.1128/aac.6.5.598. PMC 444699. PMID 15825312.
- ^ Vitale G, Amadio S, Liguori F, Volonté C (September 2024). "Empowering the NSC-34 cell line as a motor neuron model: cytosine arabinoside's action". Neural Regeneration Research. doi:10.4103/NRR.NRR-D-24-00034. PMID 39314144.
- ^ Watterson J, Toogood I, Nieder M, Morse M, Frierdich S, Lee Y, et al. (December 1994). "Excessive spinal cord toxicity from intensive central nervous system-directed therapies". Cancer. 74 (11): 3034–3041. doi:10.1002/1097-0142(19941201)74:11<3034::AID-CNCR2820741122>3.0.CO;2-O. PMID 7954266.
- ^ Chhikara BS, Parang K (December 2010). "Development of cytarabine prodrugs and delivery systems for leukemia treatment". Expert Opinion on Drug Delivery. 7 (12): 1399–1414. doi:10.1517/17425247.2010.527330. PMID 20964588. S2CID 2988492.
- ^ Hall D (November 2019). "Sea Sponges: Pharmacies of the Sea". Smithsonian Ocean. Retrieved 25 April 2023.
- ^ Perry MJ (2008). The Chemotherapy source book. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 80. ISBN 978-0-7817-7328-7.
- ^ Feist P (April 2005). "A Tale from the Sea to Ara C". Archived from the original on 2007-03-06.
- ^ Liliemark JO, Gahrton G, Paul CY, Peterson CO (June 1987). "ara-C in plasma and ara-CTP in leukemic cells after subcutaneous injection and continuous intravenous infusion of ara-C in patients with acute nonlymphoblastic leukemia". Seminars in Oncology. 14 (2 Suppl 1): 167–171. PMID 3589691.
- ^ Clarke ML, Mackey JR, Baldwin SA, Young JD, Cass CE (2002). "The Role of Membrane Transporters in Cellular Resistance to Anticancer Nucleoside Drugs". Clinically Relevant Resistance in Cancer Chemotherapy. Cancer Treatment and Research. Vol. 112. pp. 27–47. doi:10.1007/978-1-4615-1173-1_2. ISBN 978-1-4613-5428-4. PMID 12481710.
- ^ Shelton J, Lu X, Hollenbaugh JA, Cho JH, Amblard F, Schinazi RF (December 2016). "Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs". Chemical Reviews. 116 (23): 14379–14455. doi:10.1021/acs.chemrev.6b00209. PMC 7717319. PMID 27960273.
- ^ Drake JC, Hande KR, Fuller RW, Chabner BA (March 1980). "Cytidine and deoxycytidylate deaminase inhibition by uridine analogs". Biochemical Pharmacology. 29 (5): 807–811. doi:10.1016/0006-2952(80)90561-4. PMID 20227960.
- ^ Hollenbaugh JA, Shelton J, Tao S, Amiralaei S, Liu P, Lu X, et al. (Jan 2017). "Substrates and Inhibitors of SAMHD1". PLOS ONE. 12 (1): e0169052. Bibcode:2017PLoSO..1269052H. doi:10.1371/journal.pone.0169052. PMC 5207538. PMID 28046007.
- ^ Schneider C, Oellerich T, Baldauf HM, Schwarz SM, Thomas D, Flick R, et al. (February 2017). "SAMHD1 is a biomarker for cytarabine response and a therapeutic target in acute myeloid leukemia" (PDF). Nature Medicine. 23 (2): 250–255. doi:10.1038/nm.4255. PMID 27991919. S2CID 205398095.
- ^ Lemke TL, Williams DH, Foye WO (2002). Foye's principles of medicinal chemistry. Hagerstwon, MD: Lippincott Williams & Wilkins. p. 963. ISBN 0-683-30737-1.
- ^ a b Colón-Cesario M, Wang J, Ramos X, García HG, Dávila JJ, Laguna J, et al. (May 2006). "An inhibitor of DNA recombination blocks memory consolidation, but not reconsolidation, in context fear conditioning". The Journal of Neuroscience. 26 (20): 5524–33. doi:10.1523/JNEUROSCI.3050-05.2006. PMC 6675301. PMID 16707804.
- ^ Schwartsmann G, Brondani da Rocha A, Berlinck RG, Jimeno J (April 2001). "Marine organisms as a source of new anticancer agents". The Lancet. Oncology. 2 (4): 221–225. doi:10.1016/s1470-2045(00)00292-8. PMID 11905767.
- ^ Sneader W (2005). Drug discovery: a history. New York: Wiley. p. 258. ISBN 0-471-89979-8.
- ^ Ogbomo H, Michaelis M, Klassert D, Doerr HW, Cinatl J (December 2008). "Resistance to cytarabine induces the up-regulation of NKG2D ligands and enhances natural killer cell lysis of leukemic cells". Neoplasia. 10 (12): 1402–1410. doi:10.1593/neo.08972. PMC 2586691. PMID 19048119.
