ANTH domain
Appearance
(Redirected from ANTH)
| ANTH domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
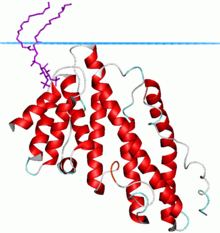 Clathrin assembly lymphoid myeloid leukemia (CALM) protein | |||||||||
| Identifiers | |||||||||
| Symbol | ANTH | ||||||||
| Pfam | PF07651 | ||||||||
| InterPro | IPR011417 | ||||||||
| OPM superfamily | 38 | ||||||||
| OPM protein | 1hfa | ||||||||
| CDD | cd03564 | ||||||||
| |||||||||
The ANTH domain is a membrane binding domain that shows weak specificity for PtdIns(4,5)P2. It was found in AP180 (homologous to CALM[1]) endocytotic accessory protein that has been implicated in the formation of clathrin-coated pits. The domain is involved in phosphatidylinositol 4,5-bisphosphate binding and is a universal adaptor for nucleation of clathrin coats.[2][3]
Its structure is a solenoid of 9 helices. The PtdIns(4,5)P2 binding residues are spread over several helices at the tip of the structure. The PtdIns(4,5)P2 binding sequence is Kx9Kx(K/R)(H/Y).
An ANTH domain is also found in HIP1 and HIP1R, and the PtdIns(4,5)P2 binding sequence is conserved.
Human proteins containing this domain
[edit]References
[edit]- ^ "Clathrin and its interactions with AP180". Archived from the original on 2007-03-11.
- ^ de Camilli P, McMahon HT, Peter BJ, Stahelin RV, Cho W, Long F, Murray D (2003). "Contrasting membrane interaction mechanisms of AP180 N-terminal homology (ANTH) and epsin N-terminal homology (ENTH) domains". J. Biol. Chem. 278 (31): 28993–9. doi:10.1074/jbc.M302865200. PMID 12740367.
- ^ Payne GS, Duncan MC (2003). "ENTH/ANTH domains expand to the Golgi". Trends Cell Biol. 13 (5): 211–5. doi:10.1016/S0962-8924(03)00076-X. PMID 12742163.
Further reading
[edit]- Ford MG, Pearse BM, Higgins MK, et al. (February 2001). "Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes". Science. 291 (5506): 1051–5. CiteSeerX 10.1.1.407.6006. doi:10.1126/science.291.5506.1051. PMID 11161218.
External links
[edit]- UMich Orientation of Proteins in Membranes protein/pdbid-1hfa - Calculated spatial position of ANTH domain of CALM protein in membrane
