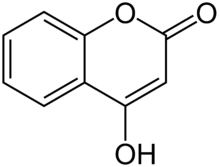
4-Hydroxycoumarin
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Hydroxy-2H-1-benzopyran-2-one | |
| Other names
4-Coumarinol
Benzotetronic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.783 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H6O3 | |
| Molar mass | 162.144 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
4-Hydroxycoumarin is a coumarin derivative with a hydroxy group at the 4-position.
Occurrence
[edit]4-Hydroxycoumarin is an important fungal metabolite from the precursor coumarin, and its production leads to further fermentative production of the natural anticoagulant dicoumarol. This happens in the presence of naturally occurring formaldehyde, which allows attachment of a second 4-hydroxycoumarin molecule through the linking carbon of the formaldehyde, to the 3-position of the first 4-hydroxycoumarin molecule, to give the semi-dimer the motif of the drug class. Dicoumarol appears as a fermentation product in spoiled sweet clover silages and is considered a mycotoxin.[1]
4-Hydroxycoumarin is biosynthesized from malonyl-CoA and 2-hydroxybenzoyl-CoA by the enzyme 4-hydroxycoumarin synthase.[2]
Anticoagulants
[edit]After the identification of dicoumarol and its anticoagulant activity, it became the prototype for a class of drugs. 4-Hydroxycoumarin forms the core of the chemical structure of anticoagulants known collectively as 4-hydroxycoumarins. They include, for example, warfarin, a pharmaceutical drug used to prevent formation of blood clots, and brodifacoum, a widely used rodenticide.
See also
[edit]References
[edit]- ^ Bye, A.; King, H. K. (1970). "The biosynthesis of 4-hydroxycoumarin and dicoumarol by Aspergillus fumigatus Fresenius". Biochemical Journal. 117 (2): 237–245. doi:10.1042/bj1170237. PMC 1178855. PMID 4192639.
- ^ Liu, B.; Raeth, T.; Beuerle, T. & Beerhues, L. (2010). "A novel 4-hydroxycoumarin biosynthetic pathway". Plant Mol. Biol. 72 (1–2): 17–25. doi:10.1007/s11103-009-9548-0. PMID 19757094. S2CID 24313989.
