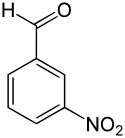
3-Nitrobenzaldehyde
Appearance
(Redirected from 3-nitrobenzaldehyde)
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3-Nitrobenzaldehyde | |||
| Other names
m-Nitrobenzaldehyde, meta-nitrobenzaldehyde
| |||
| Properties | |||
| C7H5NO3 | |||
| Molar mass | 151.121 g·mol−1 | ||
| Appearance | Yellowish to brownish crystalline powder or granulate | ||
| Melting point | 58.5 °C (137.3 °F; 331.6 K) | ||
| Boiling point | 164 °C (327 °F; 437 K) at 23 mmHg | ||
| 16.3 mg/mL | |||
| -68.55·10−6 cm3/mol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.520 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Harmful,Potentially mutagenic | ||
| GHS labelling: | |||
 
| |||
| Warning | |||
| H302, H315, H319, H335, H411 | |||
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
3-Nitrobenzaldehyde is an organic compound with the formula O2NC6H4CHO. It is one of three isomers of nitrobenzaldehyde. It contains a nitro group meta-substituted to the aldehyde.
3-Nitrobenzaldehyde is the primary product obtained via the mono-nitration of benzaldehyde with nitric acid.[3]
- C6H5CHO + HNO3 → O2NC6H4CHO + H2O
Product distribution is about 19% for the ortho-, 72% for the meta- and 9% for the para isomers.
Uses
[edit]3-Nitrobenzaldehyde is a precursor to the drug Tipranavir. It is a mainstay in the synthesis of Dihydropyridine calcium channel blockers. Via selective reduction of the nitro group, it is a precursor to the diazonium salt.[4]
References
[edit]- ^ 3-Nitrobenzaldehyde
- ^ "3-Nitrobenzaldehyde MSDS". Archived from the original on 2011-07-07. Retrieved 2009-07-18.
- ^ Brühne, Friedrich; Wright, Elaine (2011). "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3-527-30385-4.
- ^ Johannes S. Buck and Walter S. Ide (1933). "m-Chlorobenzaldehyde". Organic Syntheses. 13: 28. doi:10.15227/orgsyn.013.0028.