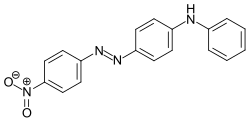
Disperse Orange 1
Appearance
(Redirected from 2581-69-3)
| |
| Names | |
|---|---|
| Other names
4-Anilino-4'-nitroazobenzene
C.I. 11080 (Colour index numbers) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.018.141 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H14N4O2 | |
| Molar mass | 318.33476 g/mol |
| Melting point | 160.0 °C (320.0 °F; 433.1 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H317 | |
| P261, P272, P280, P302+P352, P321, P333+P313, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Disperse Orange 1, or 4-anilino-4'-nitroazobenzene, is an azo dye. Commercial samples contain approximately 25% dye by weight, with the remaining mass consisting of NaCl and other salts.
This dye is useful in conducting experiments with flash photolysis due to the isomerization effect between the trans-4A4N and cis-4A4N states that occurs during photo relaxation.[1][2]
References
[edit]- ^ Hair, S. R.; Taylor, G. A.; Schultz, L. W. J. (1990). "An Easily Implemented Flash Photolysis Experiment for the Physical Chemistry Laboratory: the Isomerization of 4-Anilino-4'-Nitroazobenzene". Journal of Chemical Education. 67 (8): 709. Bibcode:1990JChEd..67..709H. doi:10.1021/ed067p709.
- ^ Wildes, P. D.; Pacifici, J. G.; Irick, G.; Whitten, D. G. J. Am. Chem. Soc., 1971, 93, 2004.
