2-Pyridone
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyridin-2(1H)-one | |||
| Other names
2(1H)-Pyridinone
2(1H)-Pyridone 1H-Pyridine-2-one 2-Pyridone 1,2-Dihydro-2-oxopyridine 1H-2-Pyridone 2-Oxopyridone 2-Pyridinol 2-Hydroxypyridine | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.019 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H5NO | |||
| Molar mass | 95.101 g·mol−1 | ||
| Appearance | Colourless crystalline solid | ||
| Density | 1.39 g/cm3 | ||
| Melting point | 107.8 °C (226.0 °F; 380.9 K) | ||
| Boiling point | 280 °C (536 °F; 553 K) decomp. | ||
| Solubility in other solvents | Soluble in water, methanol, acetone | ||
| Acidity (pKa) | 11.65 | ||
| UV-vis (λmax) | 293 nm (ε 5900, H2O soln) | ||
| Structure | |||
| Orthorhombic | |||
| planar | |||
| 4.26 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
irritating | ||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H301, H315, H319, H335 | |||
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 210 °C (410 °F; 483 K) | ||
| Related compounds | |||
Other anions
|
2-Pyridinolate | ||
Other cations
|
2-Hydroxypyridinium-ion | ||
Related functional groups
|
alcohol, lactam, lactim, pyridine, ketone | ||
Related compounds
|
pyridine, thymine, cytosine, uracil, benzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
2-Pyridone is an organic compound with the formula C
5H
4NH(O). It is a colourless solid. It is well known to form hydrogen bonded dimers and it is also a classic case of a compound that exists as tautomers.
Tautomerism
[edit]
The second tautomer is 2-hydroxypyridine. This lactam lactim tautomerism can also be exhibited in many related compounds.[1]
Tautomerism in the solid state
[edit]The amide group can be involved in hydrogen bonding to other nitrogen- and oxygen-containing species.
The predominant solid state form is 2-pyridone. This has been confirmed by X-ray crystallography which shows that the hydrogen in solid state is closer to the nitrogen than to the oxygen (because of the low electron density at the hydrogen the exact positioning is difficult), and IR-spectroscopy, which shows that the C=O longitudinal frequency is present whilst the O-H frequencies are absent.[2][3][4][5]
Tautomerism in solution
[edit]The tautomerization has been exhaustively studied. The energy difference appears to be very small. Non-polar solvents favour 2-hydroxypyridine whereas polar solvents such as alcohols and water favour the 2-pyridone.[1][6][7]
The energy difference for the two tautomers in the gas phase was measured by IR-spectroscopy to be 2.43 to 3.3 kJ/mol for the solid state and 8.95 kJ/mol and 8.83 kJ/mol for the liquid state.[8][9][10]
Tautomerisation mechanism A
[edit]The single molecular tautomerisation has a forbidden 1-3 suprafacial transition state and therefore has a high energy barrier for this tautomerisation, which was calculated with theoretical methods to be 125 or 210 kJ/mol. The direct tautomerisation is energetically not favoured. There are other possible mechanisms for this tautomerisation.[10]
Dimerisation
[edit]2-Pyridone and 2-hydroxypyridine can form dimers with two hydrogen bonds.[11]
Aggregation in the solid state
[edit]In the solid state the dimeric form is not present; the 2-pyridones form a helical structure over hydrogen bonds. Some substituted 2-pyridones form the dimer in solid state, for example the 5-methyl-3-carbonitrile-2-pyridone. The determination of all these structures was done by X-ray crystallography. In the solid state the hydrogen is located closer to the nitrogen so it could be considered to be right to call the colourless crystals in the flask 2-pyridone.[1][2][3][4][5]
Aggregation in solution
[edit]In solution the dimeric form is present; the ratio of dimerisation is strongly dependent on the polarity of the solvent. Polar and protic solvents interact with the hydrogen bonds and more monomer is formed. Hydrophobic effects in non-polar solvents lead to a predominance of the dimer. The ratio of the tautomeric forms is also dependent on the solvent. All possible tautomers and dimers can be present and form an equilibrium, and the exact measurement of all the equilibrium constants in the system is extremely difficult.[11][12][13][14][15][16][17][18][19][20]
(NMR-spectroscopy is a slow method, high resolution IR-spectroscopy in solvent is difficult, the broad absorption in UV-spectroscopy makes it hard to discriminate 3 and more very similar molecules).
Some publications only focus one of the two possible patterns, and neglect the influence of the other. For example, to calculation of the energy difference of the two tautomers in a non-polar solution will lead to a wrong result if a large quantity of the substance is on the side of the dimer in an equilibrium.
Tautomerisation mechanism B
[edit]The direct tautomerisation is not energetically favoured, but a dimerisation followed by a double proton transfer and dissociation of the dimer is a self catalytic path from one tautomer to the other. Protic solvents also mediate the proton transfer during the tautomerisation.
Synthesis
[edit]2-Pyrone can be obtained by a cyclisation reaction, and converted to 2-pyridone via an exchange reaction with ammonia:
Pyridine forms an N-oxide with some oxidation agents such as hydrogen peroxide. This pyridine-N-oxide undergoes a rearrangement reaction to 2-pyridone in acetic anhydride:[21][22][23]
In the Guareschi-Thorpe condensation cyanoacetamide reacts with a 1,3-diketone to a 2-pyridone.[12][13] The reaction is named after Icilio Guareschi and Jocelyn Field Thorpe.[14][15]
Chemical properties
[edit]Catalytic activity
[edit]2-Pyridone catalyses a variety of proton-dependent reactions, for example the aminolysis of esters. In some cases, molten 2-pyridone is used as a solvent. 2-Pyridone has a large effect on the reaction from activated esters with amines in nonpolar solvent, which is attributed to its tautomerisation and utility as a ditopic receptor. Proton transfer from 2-pyridone and its tautomer have been investigated by isotope labeling, kinetics and quantum chemical methods.[16][17][24]
Coordination chemistry
[edit]2-Pyridone and some derivatives serve as ligands in coordination chemistry, usually as a 1,3-bridging ligand akin to carboxylate.[18]
In nature
[edit]2-Pyridone is not naturally occurring, but a derivative has been isolated as a cofactor in certain hydrogenases.[19]
Environmental behavior
[edit]2-Pyridone is rapidly degraded by microorganisms in the soil environment, with a half life less than one week.[20] Organisms capable of growth on 2-pyridone as a sole source of carbon, nitrogen, and energy have been isolated by a number of researchers. The most extensively studied 2-pyridone degrader is the gram positive bacterium Arthrobacter crystallopoietes,[25] a member of the phylum Actinomycetota which includes numerous related organisms that have been shown to degrade pyridine or one or more alkyl-, carboxyl-, or hydroxyl-substituted pyridines. 2-Pyridone degradation is commonly initiated by mono-oxygenase attack, resulting in a diol, such as 2,5-dihydroxypyridine, which is metabolized via the maleamate pathway. Fission of the ring proceeds via action of 2,5-dihydroxypyridine monooxygenase, which is also involved in metabolism of nicotinic acid via the maleamate pathway. In the case of Arthrobacter crystallopoietes, at least part of the degradative pathway is plasmid-borne.[26] Pyridine diols undergo chemical transformation in solution to form intensely colored pigments. Similar pigments have been observed in quinoline degradation,[27] also owing to transformation of metabolites, however the yellow pigments often reported in degradation of many pyridine solvents, such as unsubstituted pyridine or picoline, generally result from overproduction of riboflavin in the presence of these solvents.[28] Generally speaking, degradation of pyridones, dihydroxypyridines, and pyridinecarboxylic acids is commonly mediated by oxygenases, whereas degradation of pyridine solvents often is not, and may in some cases involve an initial reductive step.[26]
Analytical data
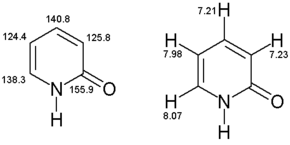
[edit]1H-NMR
[edit]1H-NMR (400 MHz, CD3OD): /ρ = 8.07 (dd,3J = 2.5 Hz,4J = 1.1 Hz, 1H, C-6), 7.98 (dd,3J = 4.0 Hz,3J = 2.0 Hz, 1H, C-3), 7.23 (dd,3J = 2.5 Hz,3J = 2.0 Hz, 1H, C-5), 7.21 (dd,3J = 4.0 Hz,4J = 1.0 Hz, 1H, C-4).
13C-NMR
[edit](100.57 MHz, CD3OD): ρ = 155.9 (C-2), 140.8 (C-4), 138.3 (C-6), 125.8 (C-3), 124.4 (C-5)
(MeOH):νmax (lg ε) = 226.2 (0.44), 297.6 (0.30).
(KBr): ν = 3440 cm−1–1 (br, m), 3119 (m), 3072 (m), 2986 (m), 1682 (s), 1649 (vs), 1609 (vs), 1578 (vs), 1540 (s), 1456 (m), 1433 (m), 1364 (w), 1243 (m), 1156 (m), 1098 (m), 983 (m), 926 (w), 781 (s), 730 (w), 612 (w), 560 (w), 554 (w), 526 (m), 476 (m), 451 (w).
EI-MS (70 eV): m/z (%) = 95 (100) [M+], 67 (35) [M+ - CO], 51 (4)[C4H3+].
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
References
[edit]- Cox RH, Bothner-By AA (1969). "Proton magnetic resonance spectra of tautomeric substituted pyridines and their conjugate acids". The Journal of Physical Chemistry. 73 (8): 2465. doi:10.1021/j100842a001.
- DW Aksnes (1972). "Substituent and solvent effects in the proton magnetic resonance (PMR) spectra of six 2-substituted pyridines" (PDF). Acta Chemica Scandinavica. 26: 2255–2266. doi:10.3891/acta.chem.scand.26-2255.
- Brügel W (1962). "Die Kernresonanzspektren von Pyridin-Derivaten". Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für physikalische Chemie. 66 (2): 159–177. doi:10.1002/bbpc.19620660211. S2CID 98754100.
- Roberts JD, Von Ostwalden PW (1971). "Nuclear magnetic resonance specroscopy. Proton spectra of 2-pyridones". The Journal of Organic Chemistry. 36 (24): 3792. doi:10.1021/jo00823a029.
See also
[edit]- 2-Pyrone
- 4-Pyridone
- The 5-methyl-2-pyridone is used to make pirfenidone.
References
[edit]- ^ a b c Forlani L., Cristoni G., Boga C., Todesco P. E., Del Vecchio E., Selva S., Monari M. (2002). "Reinvestigation of tautomerism of some substituted 2-hydroxypyridines". Arkivoc. XI (11): 198–215. doi:10.3998/ark.5550190.0003.b18. hdl:2027/spo.5550190.0003.b18.
- ^ a b Yang H. W., Craven B. M. (1998). "Charge Density of 2-Pyridone". Acta Crystallogr. B. 54 (6): 912–920. doi:10.1107/S0108768198006545. PMID 9880899. S2CID 9505447.
- ^ a b Penfold B. R. (1953). "The Electron Distribution in Crystalline Alpha Pyridone". Acta Crystallogr. 6 (7): 591–600. Bibcode:1953AcCry...6..591P. doi:10.1107/S0365110X5300168X.
- ^ a b Ohms U., Guth H., Heller E., Dannöhl H., Schweig A. (1984). "Comparison of Observed and Calculated Electron-Density 2-Pyridone, C5H5NO, Crystal-Structure Refinements at 295K and 120K, Experimental and Theoretical Deformation Density Studies". Z. Kristallogr. 169: 185–200. doi:10.1524/zkri.1984.169.14.185. S2CID 97575334.
- ^ a b Almlöf J., Kvick A., Olovsson I. (1971). "Hydrogen Bond Studies Crystal Structure of Intermolecular Complex 2-Pyridone-6-Chloro-2-Hdroxypyridine". Acta Crystallogr. B. 27 (6): 1201–1208. doi:10.1107/S0567740871003753.
- ^ Aue DH, Betowski LD, Davidson WR, Bower MT, Beak P (1979). "Gas-Phase Basicities of Amides and Imidates - Estimation of Protomeric Equilibrium-Constantes by the Basicity methode in the Gas-Phase". Journal of the American Chemical Society. 101 (6): 1361–1368. doi:10.1021/ja00500a001.
- ^ Frank J., Alan R. Katritzky (1976). "Tautomeric pyridines. XV. Pyridone-hydroxypyridine equilibria in solvents of different polarity". J Chem Soc Perkin Trans 2 (12): 1428–1431. doi:10.1039/p29760001428.
- ^ Brown R. S., Tse A., Vederas J. C. (1980). "Photoelectro-Determined Core Binding Energies and Predicted Gas-Phase Basicities for the 2-Hydroxypyridine 2-Pyridone System". Journal of the American Chemical Society. 102 (3): 1174–1176. doi:10.1021/ja00523a050.
- ^ Beak P. (1977). "Energies and Alkylation of Tautomeric Heterocyclic-Compounds - Old Problems New Answers". Acc. Chem. Res. 10 (5): 186–192. doi:10.1021/ar50113a006.
- ^ a b Abdulla H. I., El-Bermani M. F. (2001). "Infrared studies of tautomerism in 2-hydroxypyridine 2-thiopyridine and 2-aminopyridine". Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 57 (13): 2659–2671. Bibcode:2001AcSpA..57.2659A. doi:10.1016/S1386-1425(01)00455-3. PMID 11765793.
- ^ a b Hammes GG, Lillford PJ (1970). "A Kinetic and Equilibrium Study of Hydrogen Bond Dimerization of 2-Pyridone in Hydrogen Bonding Solvent". J. Am. Chem. Soc. 92 (26): 7578–7585. doi:10.1021/ja00729a012.
- ^ a b Gilchrist, T.L. (1997). Heterocyclic Chemistry ISBN 0-470-20481-8
- ^ a b Rybakov V. R., Bush A. A., Babaev E. B., Aslanov L. A. (2004). "3-Cyano-4,6-dimethyl-2-pyridone (Guareschi Pyridone)". Acta Crystallogr E. 6 (2): o160–o161. Bibcode:2004AcCrE..60O.160R. doi:10.1107/S1600536803029295.
- ^ a b I. Guareschi (1896). "Mem. Reale Accad. Sci. Torino II".
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Baron, H., Remfry, F. G. P., Thorpe, J. F. (1904). "CLXXV.-The formation and reactions of imino-compounds. Part I. Condensation of ethyl cyanoacetate with its sodium derivative". J. Chem. Soc., Trans. 85: 1726–1761. doi:10.1039/ct9048501726. Archived from the original on 2020-09-14. Retrieved 2020-06-05.
- ^ a b Fischer C. B., Steininger H., Stephenson D. S., Zipse H. (2005). "Catalysis of Aminolysis of 4-Nitrophenyl Acetate by 2-Pyridone". Journal of Physical Organic Chemistry. 18 (9): 901–907. doi:10.1002/poc.914.
- ^ a b L.-H. Wang, H. Zipse (1996). "Bifunctional Catalysis of Ester Aminolysis - A Computational and Experimental Study". Liebigs Ann. 1996 (10): 1501–1509. doi:10.1002/jlac.199619961003. Archived from the original on 2021-09-01. Retrieved 2021-09-01.
- ^ a b Rawson J. M., Winpenny R. E. P. (1995). "The coordination chemistry of 2-pyridones and its derivatives". Coordination Chemistry Reviews. 139 (139): 313–374. doi:10.1016/0010-8545(94)01117-T.
- ^ a b Shima, S.; Lyon, E. J.; Sordel-Klippert, M.; Kauss, M.; Kahnt, J.; Thauer, R. K.; Steinbach, K.; Xie, X.; Verdier, L. and Griesinger, C., "Structure elucidation: The cofactor of the iron-sulfur cluster free hydrogenase Hmd: structure of the light-inactivation product", Angew. Chem. Int. Ed., 2004, 43, 2547-2551.
- ^ a b Sims, Gerald K., S (1985). "Degradation of Pyridine Derivatives in Soil". Journal of Environmental Quality. 14 (4): 580–584. Bibcode:1985JEnvQ..14..580S. doi:10.2134/jeq1985.00472425001400040022x. Archived from the original on 2008-08-30.
- ^ "Pyridin-N-oxydと酸無水物との反應" [Reaction between Pyridin-N-oxyd and acid anhydride]. Yakugaku Zasshi (in Japanese). 67 (3–4): 51–52. 1947. doi:10.1248/yakushi1947.67.3-4_51.
- ^ Ochiai E (1953). "Recent Japanese Work on the Chemistry of Pyridine 1-Oxide and Related Compounds". The Journal of Organic Chemistry. 18 (5): 534–551. doi:10.1021/jo01133a010.
- ^ Boekelheide V, Lehn WL (1961). "The Rearrangement of Substituted Pyridine N-Oxides with Acetic Anhydride1.2". The Journal of Organic Chemistry. 26 (2): 428–430. doi:10.1021/jo01061a037.
- ^ Fischer C. B., Polborn K., Steininger H., Zipse H. (2004). "Synthesis and Solid-State Structures of Alkyl-Substituted 3-Cyano-2-pyridones" (PDF). Zeitschrift für Naturforschung. 59 (59b): 1121–1131. doi:10.1515/znb-2004-1008. S2CID 98273691. Archived from the original (subscription required) on 2008-10-30. Retrieved 2006-11-07.
- ^ Ensign JC, Rittenberg SC (1963). "A crystalline pigment produced from 2-hydroxypyridine by arthrobacter crystallopoietes n.sp". Archiv für Mikrobiologie. 47 (2): 137–153. doi:10.1007/BF00422519. PMID 14106078. S2CID 6389661.
- ^ a b Sims GK, O'Loughlin E, Crawford R (1989). "Degradation of pyridines in the environment" (PDF). CRC Critical Reviews in Environmental Control. 19 (4): 309–340. Bibcode:1989CRvEC..19..309S. doi:10.1080/10643388909388372. Archived from the original (PDF) on 2010-05-27.
- ^ Oloughlin E, Kehrmeyer S, Sims G (1996). "Isolation, characterization, and substrate utilization of a quinoline-degrading bacterium". International Biodeterioration & Biodegradation. 38 (2): 107–118. Bibcode:1996IBiBi..38..107O. doi:10.1016/S0964-8305(96)00032-7.
- ^ Sims, Gerald K., O (1992). "Riboflavin Production during Growth of Micrococcus luteus on Pyridine". Applied and Environmental Microbiology. 58 (10): 3423–3425. Bibcode:1992ApEnM..58.3423S. doi:10.1128/AEM.58.10.3423-3425.1992. PMC 183117. PMID 16348793.
Further reading
[edit]General
[edit]- Engdahl K., Ahlberg P. (1977). Journal of Chemical Research: 340–341.
{{cite journal}}: Missing or empty|title=(help) - Bensaude O, Chevrier M, Dubois J (1978). "Lactim-Lactam Tautomeric Equilibrium of 2-Hydroxypyridines. 1.Cation Binding, Dimerization and Interconversion Mechanism in Aprotic Solvents. A Spectroscopic and Temperature-Jump Kinetic Study". J. Am. Chem. Soc. 100 (22): 7055–7066. doi:10.1021/ja00490a046.
- Bensaude O, Dreyfus G, Dodin G, Dubois J (1977). "Intramolecular Nondissociative Proton Transfer in Aqueous Solutions of Tautomeric Heterocycles: a Temperature-Jump Kinetic Study". J. Am. Chem. Soc. 99 (13): 4438–4446. doi:10.1021/ja00455a037.
- Bensaude O, Chevrier M, Dubois J (1978). "Influence of Hydration upon Tautomeric Equilibrium". Tetrahedron Lett. 19 (25): 2221–2224. doi:10.1016/S0040-4039(01)86850-7.
- Hammes GG, Park AC (1969). "Kinetic and Thermodynamic Studies of Hydrogen Bonding". J. Am. Chem. Soc. 91 (4): 956–961. doi:10.1021/ja01032a028.
- Hammes GG, Spivey HO (1966). "A Kinetic Study of the Hydrogen-Bond Dimerization of 2-Pyridone". J. Am. Chem. Soc. 88 (8): 1621–1625. doi:10.1021/ja00960a006. PMID 5942979.
- Beak P, Covington JB, Smith SG (1976). "Structural Studies of Tautomeric Systems: the Importance of Association for 2-Hydroxypyridine-2-Pyridone and 2-Mercaptopyridine-2-Thiopyridone". J. Am. Chem. Soc. 98 (25): 8284–8286. doi:10.1021/ja00441a079.
- Beak P, Covington JB, White JM (1980). "Quantitave Model of Solvent Effects on Hydroxypyridine-Pyridone and Mercaptopyridine-Thiopyridone Equilibria: Correlation with Reaction-Field and Hydrogen-Bond Effects". J. Org. Chem. 45 (8): 1347–1353. doi:10.1021/jo01296a001.
- Beak P, Covington JB, Smith SG, White JM, Zeigler JM (1980). "Displacement of Protomeric Equilibria by Self-Association: Hydroxypyridine-Pyridone and Mercaptopyridine-Thiopyridone Isomer Pairs". J. Org. Chem. 45 (8): 1354–1362. doi:10.1021/jo01296a002.
Tautomerism
[edit]- Vögeli U., von Philipsborn W. (1973). "C-13 and H-1 NMR Spectroscopie Studies on Structure of N-Methyle-3-Pyridone and 3-Hydroypyridine". Org Magn Reson. 5 (12): 551–559. doi:10.1002/mrc.1270051202.
- Specker H., Gawrosch H. (1942). "Ultraviolet absorption of benztriaxole, pryridone and its salts". Chem. Ber. (75): 1338–1348. doi:10.1002/cber.19420751115.
- Leis D. G., Curran B. C. (1945). "Electric Moments of Some Gamma-Substituted Pyridines". Journal of the American Chemical Society. 67 (1): 79–81. doi:10.1021/ja01217a028.
- Albert A., Phillips J. N. (1956). "Ionisation Constants of Heterocyclic Substances Hydroxy-Derivates of Nitrogenous Six-Membered Ring-Compounds". J. Chem. Soc.: 1294–1304. doi:10.1039/jr9560001294.
- Cox R. H., Bothner-By A. A (1969). "Proton Magnetic Resonance Spectra of Tautomeric Substituted Pyridines and Their Conjugated Acides". J. Phys. Chem. 73 (8): 2465–2468. doi:10.1021/j100842a001.
- Aksnes DW, Kryvi, Kryvi H, Samuelson O, Sjöstrand E, Svensson S (1972). "Substituent and Solvent Effects in Proton Magnetic -Resonance (PMR) Spectra of 6 2-Substituted Pyridines". Acta Chem. Scand. 26 (26): 2255–2266. doi:10.3891/acta.chem.scand.26-2255.