2,5-Hexanediol
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.019.010 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H14O2 | |
| Molar mass | 118.176 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
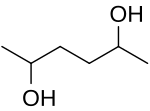
2,5-Hexanediol is an organic compound with the formula CH3CH(OH)CH2CH2CH(OH)CH3. It is both a glycol and a secondary alcohol.[1] It is a colorless water-soluble viscous liquid.[2][3][4] The chemical properties are well understood and have been extensively reported and studied.[5] It has the IUPAC name of hexane-2,5-diol[6] and the CAS Registry Number CAS 2935-44-6.[7]
Other names
[edit]- (2R,5R)-2,5-hexanediol
- 2,5-Dihydroxyhexane
- Diisopropanol
- Hexan-2,5-diol
- [R-(R*,R*)]-2,5-hexanediol
- hexane-2,5-diol
Manufacture
[edit]One common method of manufacture of the compound is from yeast.[8] Another method involves the reduction of acetonylacetone.[9] The material has two chiral carbons and thus has a number of enantiomers. Processes have been researched and developed to produce enantiopure products and by a continuous process.[10][11] Some synthesis has been carried out from keto hexanoates.[12]
Uses
[edit]One of the uses of the material is to synthesize polyesters.[13] and also fine chemicals.[14]
Toxicity
[edit]The toxicity of the material has been studied and is reasonably well understood.[15][16][17][18] It can affect the eyes and has some neurotoxic effects.[19]
References
[edit]- ^ PubChem. "2,5-Hexanediol". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-01-04.
- ^ "hexane-2,5-diol properties". materials.springer.com. Retrieved 2024-01-04.
- ^ Peter Werle; Marcus Morawietz; Stefan Lundmark; Kent Sörensen; Esko Karvinen; Juha Lehtonen (2008). "Alcohols, Polyhydric". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_305.pub2. ISBN 978-3527306732.
- ^ "2,5-Hexanediol (CAS 2935-44-6)". Cheméo. Retrieved 2024-01-04.
- ^ "2,5-Hexanediol (CAS 2935-44-6)". Cheméo. Retrieved 2024-01-04.
- ^ PubChem. "2,5-Hexanediol". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-01-04.
- ^ "2,5-Hexanediol". nist.gov.
- ^ Xiao, Meitian; Ye, Jing; Zhang, Yawu; Huang, Yayan (2009-06-01). "Reaction Characteristics of Asymmetric Synthesis of (2S,5S)-2,5-Hexanediol Catalyzed with Baker's Yeast Number 6". Chinese Journal of Chemical Engineering. 17 (3): 493–499. doi:10.1016/S1004-9541(08)60236-0. ISSN 1004-9541.
- ^ Lieser, Joan K. (July 1983). "A Simple Synthesis of (S, S)-2, 5-Hexanediol". Synthetic Communications. 13 (9): 765–767. doi:10.1080/00397918308063707. ISSN 0039-7911.
- ^ Haberland, Jürgen; Hummel, Werner; Daussmann, Thomas; Liese, Andreas (2002-07-01). "New Continuous Production Process for Enantiopure (2 R ,5 R )-Hexanediol". Organic Process Research & Development. 6 (4): 458–462. doi:10.1021/op020023t. ISSN 1083-6160.
- ^ Schroer, Kirsten; Lütz, Stephan (2009-11-20). "A Continuously Operated Bimembrane Reactor Process for the Biocatalytic Production of (2 R ,5 R )-Hexanediol". Organic Process Research & Development. 13 (6): 1202–1205. doi:10.1021/op9001643. ISSN 1083-6160.
- ^ Rudloff, E. von (1958-03-01). "SYNTHESIS OF SOME HEXANEDIOLS". Canadian Journal of Chemistry. 36 (3): 486–491. doi:10.1139/v58-069. ISSN 0008-4042.
- ^ O'Malley, James J.; Stauffer, Walter J. (April 1974). "Synthesis and characterization of isomeric polyesters based on sebacic acid and hexanediols". Journal of Polymer Science: Polymer Chemistry Edition. 12 (4): 865–874. Bibcode:1974JPoSA..12..865O. doi:10.1002/pol.1974.170120416. ISSN 0360-6376.
- ^ Herrmann, Wolfgang; Cornils, Boy; Zanthoff, Horst; Xu, Jian-He, eds. (2019-04-08). Catalysis from A to Z: A Concise Encyclopedia (1 ed.). Wiley. doi:10.1002/9783527809080.cataz09294. ISBN 978-3-527-34311-9. S2CID 219100145.
- ^ Kannan, K.; Singh, K. P.; Goel, S. K.; Shanker, Ravi (1985-02-01). "Effect of 2,5-hexanediol on immunocompetence of mice". Environmental Research. 36 (1): 14–25. Bibcode:1985ER.....36...14K. doi:10.1016/0013-9351(85)90003-9. ISSN 0013-9351. PMID 3967636.
- ^ Abou-Donia, Mohamed B.; Makkawy, H. M.; Campbell, Gerald M. (January 1985). "Pattern of neurotoxicity of n -hexane, methyl n -butyl ketone, 2,5-hexanediol, and 2,5-hexanedione alone and in combination with O -ethyl O -4-nitrophenyl phenylphosphonothioate in hens". Journal of Toxicology and Environmental Health. 16 (1): 85–100. doi:10.1080/15287398509530721. ISSN 0098-4108. PMID 4068058.
- ^ Abou-Donia, Mohamed B.; Makkawy, Hany-Anwar M.; Graham, Doyle G. (1982-03-15). "The relative neurotoxicities of n-hexane, methyl n-butyl ketone, 2,5-hexanediol, and 2,5-hexanedione following oral or intraperitoneal administration in hens". Toxicology and Applied Pharmacology. 62 (3): 369–389. doi:10.1016/0041-008X(82)90139-9. ISSN 0041-008X. PMID 7071856.
- ^ Eben, Anneliese; Flucke, Winfried; Mihail, Florin; Thyssen, Jürgen; Kimmerle, Georg (1979-06-01). "Toxicological and metabolic studies of methyl n-butylketone, 2,5-hexanedione, and 2,5-hexanediol in male rats". Ecotoxicology and Environmental Safety. 3 (2): 204–217. doi:10.1016/0147-6513(79)90012-5. ISSN 0147-6513. PMID 540560.
- ^ Jones, H. B.; Cavanagh, J. B. (1982-12-01). "Recovery from 2,5-hexanediol intoxication of the retinotectal tract of the rat". Acta Neuropathologica. 58 (4): 286–290. doi:10.1007/BF00688611. ISSN 1432-0533. PMID 6891552. S2CID 39467729.
