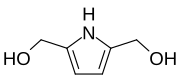
2,5-Bis(hydroxymethyl)pyrrole
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1H-Pyrrole-2,5-diyl)dimethanol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H9NO2 | |
| Molar mass | 127.143 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,5-Bis(hydroxymethyl)pyrrole is an organic chemical compound with formula C6H9O2N, or (HOCH2)2(C4H3N). Its molecule can be described as that of pyrrole C4H5N with hydroxymethyl groups HO−CH2− replacing the two hydrogen atoms adjacent to the nitrogen atom.
The compound is a white solid, soluble in water and acetone. It is stable in alkaline solutions, but otherwise tends to polymerize by self-condensation.[1] The compound was used as an intermediate in the synthesis of hexahydroporphine ("unsubstituted porphyrinogen"),[1] the core of uroporphyrinogen III, precursor of many critically important biological products such as hemoglobin and chlorophyll.
Preparation
[edit]The compound can be synthesized by formylation of pyrrole followed by reduction of the resulting pyrrolecarboxaldehyde.[1]
References
[edit]- ^ a b c Taniguchi, Shozo; Hasegawa, Hikaru; Yanagiya, Shoko; Tabeta, Yusuke; Nakano, Yoshiharu; Takahashi, Masahiko (2001). "The first isolation of unsubstituted porphyrinogen and unsubstituted 21-oxaporphyrinogen by the '3+1' approach from 2,5-bis(hydroxymethyl)pyrrole and tripyrrane derivatives". Tetrahedron. 57 (11): 2103–2108. doi:10.1016/S0040-4020(01)00059-X.
