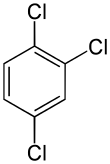
1,2,4-Trichlorobenzene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2,4-Trichlorobenzene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.026 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H3Cl3 | |||
| Molar mass | 181.44 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | aromatic[1] | ||
| Density | 1.46 g cm−3 | ||
| Melting point | 16.9 °C (62.4 °F; 290.0 K) | ||
| Boiling point | 213.5 °C (416.3 °F; 486.6 K)[2] | ||
| 0.003% (20 °C)[1] | |||
| Vapor pressure | 1 mmHg (20 °C)[1] | ||
| Related compounds | |||
Related compounds
|
1,2,3-Trichlorobenzene 1,3,5-Trichlorobenzene | ||
| Hazards | |||
| Flash point | 110 °C (230 °F; 383 K) | ||
| Explosive limits | 2.5%-6.6% (150°C)[1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
C 5 ppm (40 mg/m3)[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,2,4-Trichlorobenzene is an organochlorine compound, one of three isomers of trichlorobenzene. It is a derivative of benzene with three chloride substituents. It is a colorless liquid used as a solvent for a variety of compounds and materials.
Production and uses
[edit]Depending on the conditions and additives (e.g., sulfur), it can be the main product from the chlorination of benzene. It is virtually the exclusive product from the chlorination of 1,4-dichlorobenzene. It is also the main product from the dehydrochlorination of hexachlorocyclohexane.[3]
It is useful as a high-temperature solvent, e.g. for GPC of polyolefines such as PE or PP which are otherwise insoluble.
Aside from its use as a solvent, this compound is a useful precursor to dye and pesticides.
Safety
[edit]The LD50 (oral, rats) is 756 mg/kg. Animal studies have shown that 1,2,4-trichlorobenzene affects the liver and kidney, and is possibly a teratogen.[4] There is no regulated occupational exposure limit for chemical exposure, but the National Institute for Occupational Safety and Health recommends no greater exposure than 5 ppm, over an 8-hour workday.
See also
[edit]- Chlorobenzenes—different numbers of chlorine substituents and isomeric forms
References
[edit]- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0627". National Institute for Occupational Safety and Health (NIOSH).
- ^ Jaw, Ching-Guang; Chen, I-Ming; Yen, Jui-Hung; Wang, Yei-Shung (December 1999). "Partial solubility parameters of chlorobenzene and chlorophenol compounds at equilibrium distribution in two immiscible phases". Chemosphere. 39 (15): 2607–2620. doi:10.1016/s0045-6535(99)00173-3. ISSN 0045-6535.
- ^ Beck, U.; Löser, E. "Chlorinated Benzenes and other Nucleus-Chlorinated Aromatic Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o03. ISBN 978-3527306732.
- ^ CDC - NIOSH Pocket Guide to Chemical Hazards