Punicalagin
Appearance
(Redirected from (α/β)-punicalagin)
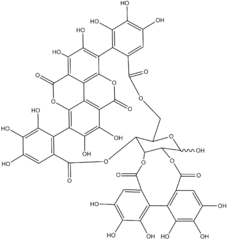
| |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C48H28O30 | |
| Molar mass | 1084.71 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Punicalagin (Pyuni-cala-jen) is an ellagitannin, a type of phenolic compound. It is found as alpha and beta isomers in pomegranates (Punica granatum), Terminalia catappa, Terminalia myriocarpa,[1] and in Combretum molle, the velvet bushwillow, a plant species found in South Africa.[2] These three genera are all Myrtales and the last two are both Combretaceae.
Research
[edit]Punicalagins are water-soluble and hydrolyze into smaller phenolic compounds, such as ellagic acid.
There were no toxic effects in rats on a 6% diet of punicalagins for 37 days.[3] In laboratory research, punicalagins had carbonic anhydrase inhibitor activity.[4]
See also
[edit]References
[edit]- ^ Marzouk, M. S. A.; El-Toumy, S. A. A.; Moharram, F. A.; Shalaby, N. M.; Ahmed, A. A. (2002). "Pharmacologically Active Ellagitannins from Terminalia myriocarpa". Planta Medica. 68 (6): 523–527. doi:10.1055/s-2002-32549. PMID 12094296.
- ^ Asres, K.; Bucar, F.; Knauder, E.; Yardley, V.; Kendrick, H.; Croft, S. L. (2001). "In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle". Phytotherapy Research. 15 (7): 613–617. doi:10.1002/ptr.897. PMID 11746844. S2CID 24511496.
- ^ Cerdá, B; Cerón, J. J; Tomás-Barberán, F. A; Espín, J. C (2003). "Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic". Journal of Agricultural and Food Chemistry. 51 (11): 3493–501. doi:10.1021/jf020842c. PMID 12744688.
- ^ Satomi, H.; Umemura, K.; Ueno, A.; Hatano, T.; Okuda, T.; Noro, T. (1993). "Carbonic anhydrase inhibitors from the pericarps of Punica granatum L". Biological & Pharmaceutical Bulletin. 16 (8): 787–790. doi:10.1248/bpb.16.787. PMID 8220326.
