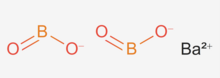
Barium borate
| |
| Names | |
|---|---|
| Other names
barium diborate, barium boron oxide, barium metaborate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.824 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BaB2O4 or Ba(BO2)2 | |
| Molar mass | 222.95 |
| Appearance | white powder or colorless crystals |
| Odor | odorless |
| Density | 3.85 g/cm3[1] |
| Melting point | 1,095 °C (2,003 °F; 1,368 K)[2] |
| Solubility in hydrochloric acid | soluble |
Refractive index (nD)
|
ne = 1.5534, no = 1.6776 |
| Structure | |
| Rhombohedral, hR126[3] | |
| R3c, No. 161 | |
a = 1.2529 nm, c = 1.274 nm
| |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302 | |
| P264, P270, P301+P312, P330, P501 | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Barium borate is an inorganic compound, a borate of barium with a chemical formula BaB2O4 or Ba(BO2)2. It is available as a hydrate or dehydrated form, as white powder or colorless crystals. The crystals exist in the high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.
Barium borate was discovered and developed by Chen Chuangtian and others of the Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences.
Properties
[edit]

Barium borate exists in three major crystalline forms: alpha, beta, and gamma. The low-temperature beta phase converts into the alpha phase upon heating to 925 °C. β-Barium borate (BBO) differs from the α form by the positions of the barium ions within the crystal. Both phases are birefringent, however the α phase possesses centric symmetry and thus does not have the same nonlinear properties as the β phase.[4]
Alpha barium borate, α-BaB2O4 is an optical material with a very wide optical transmission window from about 190 nm to 3500 nm. It has good mechanical properties and is a suitable material for high-power ultraviolet polarization optics.[5] It can replace calcite, titanium dioxide or lithium niobate in Glan–Taylor prisms, Glan–Thompson prisms, walk-off beam splitters and other optical components. It has low hygroscopicity, and its Mohs hardness is 4.5. Its damage threshold is 1 GW/cm2 at 1064 nm and 500 MW/cm2 at 355 nm.[1]
Beta barium borate, β-BaB2O4, is a nonlinear optical material transparent in the range ~190–3300 nm. It can be used for spontaneous parametric down-conversion. Its Mohs hardness is also 4.5.[1][2] The material exhibits a melting temperature of 1268 K,[6] with anisotropic thermal expansion coefficients: and α₃₃ = 36 × 10⁻⁶ K⁻¹.[7]
Gamma barium borate, γ-BaB2O4, discovered recently, was produced by heating beta barium borate 900 °C under 3 GPa of pressure. It was found to have a monoclinic crystal structure.[8]
Barium borate has strong negative uniaxial birefringence and can be phase-matched for type I (ooe) second-harmonic generation from 409.6 to 3500 nm. The temperature sensitivity of the indices of refraction is low, leading to an unusually large (55 °C) temperature phase-matching bandwidth.[2]
Although the ambient-pressure α and β crystal phases contain only trigonal, sp2 hybridized, boron, BBO glass has around 40% of the boron on tetrahedral, sp3 hybridized, sites. In the liquid state the relative fractions of sp2 and sp3 boron are temperature-dependent, with the trigonal planar coordination favored at higher temperatures.[9]
Synthesis
[edit]Barium borate can be prepared by reaction of an aqueous solution of boric acid with barium hydroxide. The prepared γ-barium borate contains water of crystallization that can not be completely removed by drying at 120 °C. Dehydrated γ-barium borate can be prepared by heating to 300–400 °C. Calcination at about 600–800 °C causes complete conversion to the β form. BBO prepared by this method does not contain trace amounts of BaB2O2[10]
BBO crystals for nonlinear optics can be grown from fluxed melt of barium borate, sodium oxide and sodium chloride.[11]
Thin films of barium borate can be prepared by MOCVD from barium(II) hydro-tri(1-pyrazolyl)borate. Different phases can be obtained depending on deposition temperatures.[12] Thin films of beta-barium borate can be prepared by sol-gel synthesis.[13]
Barium borate monohydrate is prepared from the solution of barium sulfide and sodium tetraborate. It is a white powder. It is used as an additive to e.g. paints as flame retardant, mold inhibitor, and corrosion inhibitor. It is also used as a white pigment.
Barium borate dihydrate is prepared from the solution of sodium metaborate and barium chloride at 90–95 °C. After cooling to room temperature, white powder is precipitated. Barium borate dihydrate loses water at above 140 °C. It is used as a flame retardant for paints, textiles, and paper.[14]
Applications
[edit]BBO is a popular nonlinear optical crystal. Quantum linked photons are producible with beta barium borate. Barium borate is a bactericide and fungicide.[15] It is added to paints, coatings, adhesives, plastics, and paper products.
Barium borate is resistant to ultraviolet radiation. It can act as UV stabilizer for polyvinyl chloride.[16]
The solubility of barium borate is a disadvantage when used as a pigment. Silica-coated powders are available. The alkaline properties and the anodic passivation properties of the borate ion enhance the anticorrosion performance. Commonly available barium metaborate pigment comes in three grades; Grade I is a barium metaborate itself, grade II is compounded with 27% zinc oxide, and grade III is compounded with 18% of zinc oxide and 29% calcium sulfate. Barium borate shows synergistic performance with zinc borate.[17]
Barium borate is used as a flux in some barium titanate and lead zirconate EIA Class 2 dielectric ceramic formulations for ceramic capacitors, in amount of about 2%. The barium-boron ratio is critical for flux performance; BaB2O2 content adversely affects the performance of the flux.[10][18]
Barium borate-fly ash glass can be used as radiation shielding. Such glasses are superior in performance to concrete and to other barium borate glasses.[19]
References
[edit]- ^ a b c Barium Borate (a-BBO) Crystal. casix.com
- ^ a b c BBO Crystals – Beta Barium Borate and Lithium Borate Archived February 12, 2012, at the Wayback Machine. clevelandcrystals.com
- ^ Guiqin, Dai; Wei, Lin; An, Zheng; Qingzhen, Huang; Jingkui, Liang (1990). "Thermal Expansion of the Low-Temperature Form of BaB2O4". Journal of the American Ceramic Society. 73 (8): 2526–2527. doi:10.1111/j.1151-2916.1990.tb07626.x.
- ^ Nikogosyan, D. N. (1991). "Beta barium borate (BBO)". Applied Physics A. 52 (6): 359–368. Bibcode:1991ApPhA..52..359N. doi:10.1007/BF00323647. S2CID 101903774.
- ^ Alpha Barium Borate. Roditi.com. Retrieved on 2012-01-15.
- ^ Nikogosyan, David (2005). "Chapter 2: Basic Nonlinear Optical Crystals". Nonlinear Optical Crystals: A Complete Survey (1st ed.). Springer New York, NY. p. 6. ISBN 9780387220222.
- ^ Trento, Chin (Oct 18, 2024). "From Structure to Application: Is BIBO or BBO the Better Crystal?". Stanford Advanced Materials. Retrieved Nov 8, 2024.
- ^ Bekker, Tatyana B.; Podborodnikov, Ivan V.; Sagatov, Nursultan E.; Shatskiy, Anton; Rashchenko, Sergey; Sagatova, Dinara N.; Davydov, Alexey; Litasov, Konstantin D. (2022). "γ-BaB2O4: High-Pressure High-Temperature Polymorph of Barium Borate with Edge-Sharing BO4 Tetrahedra". Inorganic Chemistry. 61 (4). ACS Publications: 2340–2350. doi:10.1021/acs.inorgchem.1c03760. PMID 35040639. S2CID 246054700.
- ^ Alderman, Oliver; Benmore, Chris; Holland, Diane; Weber, Rick (2023). "Boron coordination change in barium borate melts and glasses and its contribution to configurational heat capacity, entropy, and fragility". Journal of Chemical Physics. 158 (22): 224501. Bibcode:2023JChPh.158v4501A. doi:10.1063/5.0153282. PMID 37290074.
- ^ a b US 4897249, Ross, Sidney D., "Barium borate preparation", issued Jan 30, 1990
- ^ Gualtieri, Devlin M.; Chai, Bruce H. T. "High temperature solution growth of barium borate (BaB2O4)" U.S. patent 4,931,133 issued June 5, 1990
- ^ Malandrino, G.; Lo Nigro, R.; Fragalà, I. L. (2007). "An MOCVD Route to Barium Borate Thin Films from a Barium Hydro-tri(1-pyrazolyl)borate Single-Source Precursor". Chemical Vapor Deposition. 13 (11): 651. doi:10.1002/cvde.200706611.
- ^ C. Lu; S. S. Dimov & R. H. Lipson (2007). "Poly(vinyl pyrrolidone)-Assisted Sol−Gel Deposition of Quality β-Barium Borate Thin Films for Photonics Applications". Chem. Mater. 19 (20): 5018. doi:10.1021/cm071037m.
- ^ Perry, Dale L.; Phillips, Sidney L., eds. (1995). Handbook of inorganic compounds. Boca Raton: CRC Press. p. 3. ISBN 978-0-8493-8671-8.
- ^ Henry Warson; C. A. Finch (2001). Applications of Synthetic Resin Latices: Latices in surface castings : emulsion paints. John Wiley and Sons. pp. 885–. ISBN 978-0-471-95461-3. Retrieved 15 January 2012.
- ^ Koskiniemi, Mark S "Calcium pyroborate as a microbicide for plastics" U.S. patent 5,482,989 issued 01/09/1996
- ^ J. V. Koleske (1995). Paint and coating testing manual: fourteenth edition of the Gardner-Sward handbook. Vol. 17. ASTM International. ISBN 978-0-8031-2060-0.
- ^ K. Singh; Indurkar, Aruna (1988). "Dielectrics of lead zirconate bonded with barium borate glass" (PDF). Bull. Mater. Sci. 11: 55. doi:10.1007/BF02744501. S2CID 97981458.
- ^ Singh, Sukhpal; Kumar, Ashok; Singh, Devinder; Thind, Kulwant Singh; Mudahar, Gurmel S. (2008). "Barium-borate-flyash glasses : As radiation shielding materials". Nuclear Instruments and Methods in Physics Research Section B. 266 (1): 140. Bibcode:2008NIMPB.266..140S. doi:10.1016/j.nimb.2007.10.018.


