α-Halo ketone

In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.[1]
Structure
[edit]The general structure is RR′C(X)C(=O)R where R is an alkyl or aryl residue and X any one of the halogens. The preferred conformation of a halo ketone is that of a cisoid with the halogen and carbonyl sharing the same plane as the steric hindrance with the carbonyl alkyl group is generally larger.[2]
Halo ketone synthesis
[edit]Halo ketones and halo carbonyl compounds in general are synthesized by reaction of carbonyl compounds with sources of X+ (X = halogen), which is provided using halogens:[1]
- RC(O)CH3 + X2 → RC(O)CH2X + HX
Specialized sources of electrophilic halogenating agents include N-Bromosuccinimide and 1,3-dibromo-5,5-dimethylhydantoin (DBDMH). In the Nierenstein reaction an acyl chloride reacts with diazomethane
Asymmetric synthesis
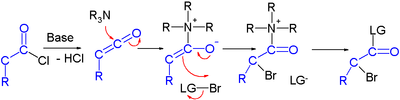
[edit]Efforts are reported in asymmetric synthesis of halo carbonyls through organocatalysis. In one study an acid chloride is converted into an α-halo ester with a strong base (sodium hydride), a bromine donor and an organocatalyst based on proline and quinine:[3]

In the proposed reaction mechanism the base first converts the acid chloride to the ketene, the organocatalyst then introduces chirality through its quinonoid tertiary amine, forming a ketene adduct.
Reactions
[edit]Illustrative of their alkylating activity are reactions with potassium iodide in acetone, chloroacetone reacts faster than 1-chloropropane by a factor of 36,000. Halo ketones react with phosphites in the Perkow reaction.
The halo group can be removed in reductive dehalogenation of halo ketones. α-Halo ketones can also be converted to alkenes by treatment with hydrazine.
Due to the presence of two electron withdrawing groups (carbonyl and halide), the α-hydrogen is acidic. This property is exploited in the Favorskii rearrangement, where base abstracts first an acidic α-hydrogen and the resulting carbanion then displaces the halogen.
In crossed aldol reactions between halo ketones and aldehydes, the initial reaction product is a halohydrin which can subsequently form an oxirane in the presence of base.
α-Halo ketones can react with amines to form an α-halo imine, which can be converted back to the parent halo ketone by hydrolysis, so that halo imines may be used as masked versions of halo ketones. This allows some chemical transformations to be achieved that are not possible with the parent halo ketones directly.[4]
Precursors to heterocycles
[edit]Halo ketones take part in several reaction types, especially since they are bifunctional, with two electrophilic sites (α-carbon and carbonyl carbon). In one manifestation of this duality, they are precursors to heterocycles. Thiazoles arise from reaction of chloroacetone with thioamides.2-Aminothiazoles are similarly produced by reaction of 2-chloroketones with thioureas.[5][6] Pyrroles may be synthesized by reaction of halo ketones with dicarbonyls and ammonia in the Hantzsch pyrrole synthesis.
References
[edit]- ^ a b Verhé, Roland; De Kimpe, Norbert (1983). "Synthesis and Reactivity of α-Halogenated Ketones". In Saul Patai, Zvi Rappoport (ed.). Halides, Pseudo-Halides and Azides: Vol. 1. PATAI'S Chemistry of Functional Groups. pp. 813–931. doi:10.1002/9780470771716.ch19. ISBN 9780470771716.
- ^ Erian, Ayman W.; Sherif, Sherif M.; Gaber, Hatem M. (2003). "The Chemistry of α-Haloketones and Their Utility in Heterocyclic Synthesis" (PDF). Molecules. 8 (11): 793–865. doi:10.3390/81100793. S2CID 53951565.
- ^ Dogo-Isonagie, Cajetan; Bekele, Tefsit; France, Stefan; Wolfer, Jamison; Weatherwax, Anthony; Taggi, Andrew E.; Lectka, Thomas (2006). "Scalable Methodology for the Catalytic, Asymmetric α-Bromination of Acid Chlorides". Journal of Organic Chemistry. 71 (23): 8946–8949. doi:10.1021/jo061522l. PMID 17081026.
- ^ Verhé, Roland; De Kimpe, Norbert (1983). "α-Halogenated Imines". In Saul Patai, Zvi Rappoport (ed.). Halides, Pseudo-Halides and Azides: Vol. 1. PATAI'S Chemistry of Functional Groups. pp. 813–931. doi:10.1002/9780470771716.ch13. ISBN 9780470771716.
- ^ J. R. Byers; J. B. Dickey (1939). "2-Amino-4-methylthiazole". Organic Syntheses. 19: 10. doi:10.15227/orgsyn.019.0010.
- ^ George Schwarz (1945). "2,4-Dimethylthiazole". Organic Syntheses. 25: 35. doi:10.15227/orgsyn.025.0035.
